Transcription
Cryo-electron microscopy at LeedsApproaches and challenges for highresolution data acquisitionDr Rebecca Thompson
Officially openedJanuary 2017.
Transmission electronmicroscopes JEOL 1400- Negative stain/ambient sections FEI T12- Negative stain/ambient sections FEI F20- Negative stain/ambient sections/ cryo-EMscreening/tomography
Sample preparation of macromolecular complexes Leica EM-GP- adherentcells/macromolecular complexes Vitrobot Mk 4- macromolecularcomplexes Housed in 20 % RH samplepreparation room
Spraying approaches and timeresolved EMCryo-EM data collectionStephen Muench, Howard White
Sample preparation of cells, tissues and organisms New this year! Leica EM ICE High pressure freezer- forvitrification of cells, tissues andorganisms like C. Elegans Leica AFS2 for freeze substitution ofhigh pressure frozen specimens Leica UC7 cryo/ultramicrotome forsectioning ofambient/Tokyasu/vitreous (CEMOVIS)samples
Glow dischargeQuorum GloQubeCressington 208Considering purchase of a plasma cleaner- opinions welcome!PELCO easiGlow
Cryo-FluorescenceLeica CryoCLEM systemLinkam cryo fluorescence stage(Can be fitted onto Zeiss LSM 880 with Airyscan)
Facility operates at BSL2
Rebecca ThompsonFacility Manager/ Senior cryo-EMscientistThe TeamDan MaskellCryo-EM support scientistTraining LeadEmma HeskethCryo-EM support scientistIT leadMartin FullerEM technicianJEOL1400/T12 and Ultramicrotomy lead
The User Base 134 ‘active’ users from across the University 75 % internal users Faculty Biological Sciences, with remainder from Chemistry,Physics, Medicine, Food Science, Engineering/Materials Science, and externalcollaborators. 25-30 use cryo-EM as primary tool of research On Titan Krioses, 20 % available time used by external service users
External Users Mix of industry and academia Shortly to offer access through Instruct-ERIC 75 % attend site for collection (repeat customers mostlikely not to attend Remote customers can see files for inspection duringcollection, other feedback done via skype and email. Plansto implement remove viewing. Do not offer remote control (and no plans for users)
Equipment Access Managed through Instruct’s ‘Aria’booking system Titan Krios bookings managed byfacility staff Balance of internal/external,projects and unfunded currentlymanaged by Facility staff withoversight from Director
Standard Single Particle Pipelines
Sample optimizationand screening is amassive bottleneckWelldistributed We end up usingKrioses for (most)screening Aim to make this asefficient as possible2/3 loads perscreening day (22-33grids) plus‘overnight’ shortruns for borderlineprojectsAffinity forthe supportThin ice incenter of holepushingparticles tothe hole edgePreferredorientation atair waterinterfaceDrulyte et al 2018
Data collection
E Plubirus Paucioribus?(Out of Many, Fewer)Sarkar et al, NSBM 2017
Data collection- Facility staff How can we as Support Scientists provide best possible service? Monitoring of equipment and environment (26 parameters measured and alarmed) Work to an SOP (but when providing service will deviate if requested) Checklists How can we best monitor performance of the microscopes over time? Periodic collection of ‘standard’ samples? How do you (continually) train cryo-EM support staff?
Training ModuleInductionsInduction - Service labInduction - Cryo labModuleCode000001002Grid Prep - StainCarbon coating "Collodionmethod"Carbon coating "floating"Glow discharge CresingtonGlow discharge Ted PellaGlow discharge GloCubeNegative staining101a101b102a102b102c103Grid prep - cryoVitrobotLeica GP200201a201bMicroscope TrainingJEOLT12 - beginnersT12 - advancedF20Krios 1 - EPUKrios 2 - EPUKrios 1 - Advanced usersKrios 2 - Advanced er packBasic Unix commandsGeneral computing guideRelion starters guidecryo sparcUsing Arc3Working from homeAccess screening data - KriosOTF - internal400401401a401b401c401d401e401f401g402H&S Induction(1h)100Negativestain/T12( 7h) 20 h experienceF20 training ( 2 hambient, 20 h cryo)Cryo grid prep(3 h)Titan Kriosscreeningtraining (812 h, 1-1)Titan KriosEPU training(8-12 h 1-1)
Data collection- internal usersWhich detector?Detectormode?How manyimages will Iget?Total dose?Dose per frame?Phase plate?Mag?Acquisitionsper hole?How many do Ineed?Energyfilter?Just HOW do I do it?
Training ModuleInductionsInduction - Service labInduction - Cryo labModuleCode000001002Grid Prep - StainCarbon coating "Collodionmethod"Carbon coating "floating"Glow discharge CresingtonGlow discharge Ted PellaGlow discharge GloCubeNegative staining100101a101b102a102b102c103Grid prep - cryoVitrobotLeica GP200201a201bMicroscope TrainingJEOLT12 - beginnersT12 - advancedF20Krios 1 - EPUKrios 2 - EPUKrios 1 - Advanced usersKrios 2 - Advanced er packBasic Unix commandsGeneral computing guideRelion starters guidecryo sparcUsing Arc3Working from homeAccess screening data - KriosOTF - internal400401401a401b401c401d401e401f401g402
Teaching aid Resource for external users Improves reproducibility Reduces sessions ’lost’ forpreventable reasons Helps with trouble shooting Users require less expertknowledge- but still need tounderstand why they are makingchoices! Reduced appetite for becoming acryo-EM ‘expert’- learning on a‘need to know’ basisIn Press Nature Protocols
User data sheet provided for everysession Improves tracking of parameters Reduces users errors duringprocessing
Detector choice
Which Detector? Internal users split pretty evenly acrossF3EC and K2 F3EC used mostly in integrating mode Tomography/sub 120-150 kDa singleparticle projects K2 Internal users never really use K2 superresolution External users preference for K2counting
2.6 Å structure fromFalcon III in integratingmode 48 h data collection 5619 Micrographs (117/hour) Autopicked 300,000 280,000 particles in finalreconstructionEmma Hesketh
Falcon 3 in integratingmode 200 kDa soluble protein 4267 micrographs Autopicked 1.6 million particles 110 e/A2 so you can see theparticles! 3.5 Å structureIeva Drulyte
Dealing with the Data Deluge
‘Current’ OTFimage processing
Assessing Micrograph QualityBad dataset‘Requires investigation’ datasetMatt Iadanza
Outlook Efficient screening continues to be a challenge- Focused screening days andovernight collections for ‘borderline’ projects. New hardware may help. Training and democratization of knowledge- how can we share best practice? Single OTF processing changing rapidly- challenge to constantly adapt systems Expect tomography work to increase- implementing OTF pipelines for assessingdata (especially PP) Implementation of other software for routine collection (SerialEM) oftomography data, not just dose-symmetric schemes.
AcknowledgementsNeil RansonEmma HeskethMartin FullerDan MaskellMatt IadanzaShaun RawsonIeva Drylute
Thank you for your attentionAny questions?@AstburyBSL@Bex 16
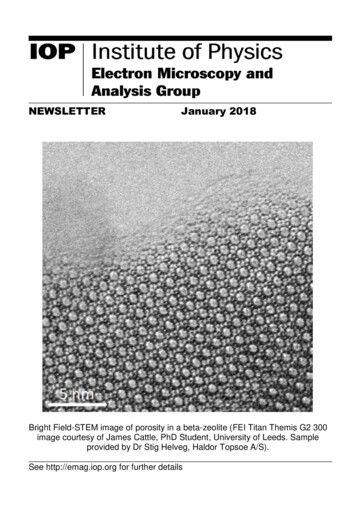
Cryo-electron microscopy at Leeds Approaches and challenges for high resolution data acquisition . Officially opened January 2017. Transmission electron microscopes JEOL 1400- Negative stain/ambient sections FEI T12-Negative stain/ambient sections FEI F20-Negative stain/ambient sections/ cryo-EM screening/tomography. Sample .