Transcription
Published on 18 July 2014. Downloaded by Secretaria General Adjunta de Informatica (SGAI) on 7/31/2019 8:22:53 AM.RSC AdvancesView Article OnlineREVIEWView Journal View IssueSolid-phase peptide synthesis: an overview focusedon the preparation of biologically relevant peptidesCite this: RSC Adv., 2014, 4, 32658Jose M. Palomo*Received 20th March 2014Accepted 18th July 2014This review article highlights the strategies to successfully perform an efficient solid-phase synthesis ofcomplex peptides including posttranslational modifications, fluorescent labels, and reporters or linkinggroups of exceptional value for biological studies of several important diseases. The solid-phaseapproach is the best alternative to synthesize these peptides rapidly and in high amounts. The keyDOI: 10.1039/c4ra02458caspects that need to be considered when performing a peptide synthesis in solid phase of thesewww.rsc.org/advancesmolecules are discussed.1. IntroductionPeptide synthesis has been, for more than a century, a signi cant synthetic approach in different areas such as proteinchemistry and organic synthesis. Basically, the peptide formation goes through a repetitive amidation reaction between anamino group of one amino acid and the carboxylic group of asecond one, the so-called peptide bond. In Nature these interactions between the twenty natural L-amino acids nallyproduce proteins. Fischer demonstrated that proteins areformed by amino acids and the peptide bond the interactionbetween them. He synthesized the rst peptide (glycyl–glycine)and coined the name1 being considered the “father” of thepeptide chemistry. Fischer's former student, Max Bergmann,introduced the carbobenzoxy group for the suitable reversibleblocking group for the amine function which ushered in a newera.2 This general scheme was universally in use for years and itwas very effective, for example, for the rst synthesis of apeptide hormone by du Vigneaud in 1953.3Nowadays, one of the main applications of Fischer'sdiscovery is to create semisynthetic proteins,4 natural or nonnatural ones, to aid in understanding biological processes5(Fig. 1). In particular, the posttranslational modi cations areessential for protein activation: phosphorylation for signaltransduction, ubiquitination for proteolysis, attachment of fattyacids for membrane anchoring or glycosylation for extendingprotein half-life, targeting, and cell–cell interactions.6The availability of a certain amount (milligrams) of thesetypes of proteins (incorporating one or several posttranslationalmodi cations) is mandatory for biological studies of a particular disease. However, the production of these target proteins inpure form is extremely complex or in some cases evenDepartamento of Biocatalisis, Instituto de Catalisis (CSIC), Marie Curie 2,Cantoblanco 28049, Madrid, Spain. E-mail: josempalomo@icp.csic.es; Fax: 34915854760; Tel: 34-91585476832658 RSC Adv., 2014, 4, 32658–32672unfeasible by biochemical methods. Therefore, the workdeveloped in peptide synthesis in the last years has permittedthe successful preparation of tailor-made peptide sequencescarrying out posttranslational changes, uorescent labels,etc.4b,7,8 These designed peptides have been linked to the engineered target protein a erwards – combining chemical andbiological methods – to create natural proteins or semisyntheticnon-natural analogues.7,8The giant step for the evolution in the synthesis of largerand more complex peptides (or even proteins)9 has been madepossible by the solid-phase. Bruce Merri eld was the pioneer ofthe idea of generating peptide bonds on an insoluble resinwhere a single cleavage step permits obtaining the desiredpeptide in solution.10 This won him the Nobel Prize inChemistry in 1984.11 The principle advantages of the chemistryon solid-phase are: (i) simplicity and speed (carrying out all thereactions in a single reaction vessel) and (ii) efficiency(avoiding the large losses which normally are encounteredduring the isolation and puri cation of intermediates,obtaining high yields of nal products through the use ofexcess reactants).The strategies developed for peptide synthesis in solutionhave been adapted to the solid phase, although in this type ofthe synthetic approach several issues such as the matrixswelling, type of linker groups, protecting groups in the aminoacid residues, etc. must be considered. For the last twenty years,researchers have developed strategies to improve the performance in solid-phase peptide synthesis (SPPS). The applicationof microwave systems, the development of novel supports ornovel activating groups are some of the emerging systems,which has been the focus of recent review articles.12–14In this article, a general view of peptide synthesis on solidphase and how this protocol has been successfully applied inthe preparation of different important peptide sequences tostudy biological processes is emphasized.This journal is The Royal Society of Chemistry 2014
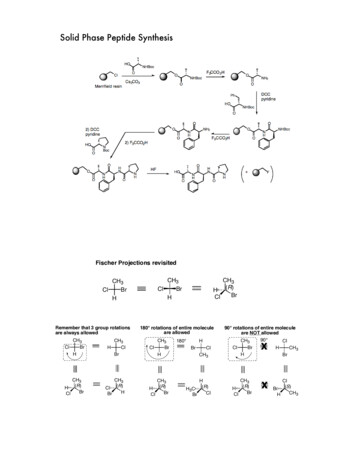
View Article OnlinePublished on 18 July 2014. Downloaded by Secretaria General Adjunta de Informatica (SGAI) on 7/31/2019 8:22:53 AM.ReviewFig. 1RSC AdvancesExamples of semisynthetic biological proteins created by tailor-made peptide synthesis.2. Solid-phase peptide synthesis(SPPS)2.1 Initial aspects of the peptide synthesis: N-terminalamino acid protecting groupThe general process for synthesizing peptides on a resin startsby attaching the rst amino acid (AA), from the C-terminalresidue (carboxyl group), then proceeding with the peptidesequence construction to the N-terminal end. The amino acidsare coupled to the supported peptide sequence by the alphaamino group and the reactive side chains are protected by atemporary protecting group. The carboxyl group of the corresponding amino acid must be activated for its coupling to theresin. Once the amino acid is attached, the resin is ltered andwashed to remove byproducts and excess reagents. Next, the Na-protecting group of the new couple AA is removed (deprotection process) and the resin is again washed to removebyproducts and excess reagents. Then, the next amino acid iscoupled to the attached one. The cycle is repeated until thepeptide sequence is complete. Then typically, all the protectinggroups are removed, the resin is washed and the peptide iscleaved from the resin (Scheme 1).A feature that de nes the chemistry in the SPPS is thetemporary protecting group in the amino acid N-terminalamino group. The two most widely used are the tert-butoxycarbonyl (Boc) group (sensitive to acids such as tri uoroaceticacid (TFA)),10,15 and the uoren-9-ylmethyloxycarbonyl (Fmoc)group (sensitive to bases such as piperidine) (Fig. 2).16The Fmoc strategy is o en preferred over the Boc strategy forroutine synthesis, because the former can be removed undermilder conditions than the latter, representing an orthogonaldeprotection scheme considering that most of linkers andprotecting groups of amino acid residues can be deprotectedunder acidic conditions.17 However, the application of an in situneutralization protocol15 has made the BOC chemistry theselected strategy for increasing the efficiency in difficult orlonger peptide sequences even implemented in automatedsynthesizers.The coupling of the rst amino acid represents a key step onan efficient SPPS, where a nearly quantitative yield is mandatory. The yield and scale of the solid-phase reactions are givenScheme 1This journal is The Royal Society of Chemistry 2014General scheme of solid-phase peptide synthesis (SPPS).RSC Adv., 2014, 4, 32658–32672 32659
View Article OnlinePublished on 18 July 2014. Downloaded by Secretaria General Adjunta de Informatica (SGAI) on 7/31/2019 8:22:53 AM.RSC AdvancesReviewScheme 2Reaction mechanism of peptides with ninhydrin (Kaisertest).Deprotection scheme of the most popular protecting groups ofa-amino groups. (A) Boc deprotection mechanism. (B) Fmoc deprotection mechanism.Fig. 2with respect to the amount of this rst amino acid coupled ontothe resin. Loading in the typical commercial resins goes from0.3 to 2.5 mmol(reactive group) per g(support).The Fmoc-group has the advantage that can be used tomonitor the rst amino acid coupling to the resin by simple UVspectrophotometry analysis (Fig. 2).18 Several milligrams of dryAA-resin can be treated with a solution of 20% piperidine inDMF for 30 min, and then (not later) this solution (3 mL) can beused for the monitoring of the UV absorbance at 301 nm of thedibenzofulvene-piperidine adduct formed (Fig. 2). The samepiperidine solution must be used as a reference for the previouscalibration of the UV spectrometer to zero. The loading can becalculated by using this equation:18 loading (mmol g 1) ¼Abssample,301nm 0.4*, (* based on 3 ¼ 6000 M 1 cm 1); 3 mLdeblocking solution, 5 mg resin, UV cell 10 mm.182.2Monitoring of coupling and cappingMonitoring of the coupling steps is critical in the successfulpeptide synthesis. Different tests have been developed for thatpurpose.19–21The Kaiser test is historically and today one of the mostused.19 It is a qualitative test for the presence or absence of freeprimary amino groups, and it can be a useful indicator of thecompleteness of a coupling step. The test is based on thereaction of ninhydrin19 with primary amines, which gives a32660 RSC Adv., 2014, 4, 32658–32672characteristic dark blue color (Scheme 2). For determining thepresence of secondary amine the choranil/acetaldehyde test21 isthe most adequate.In addition, the tests require minimal amounts of analyteand it is completed within a few minutes. The testing protocolshould be followed carefully. Excess heat can cause loss of Fmocprotecting groups through reaction with pyridine (contained inthe test reagents), resulting in a false positive result. If themonitoring test indicates the presence of unreacted aminefollowing a coupling reaction, a second coupling step or themodi cation of the coupling conditions should be performed.If it is still present a er this treatment, the capping of the aminogroup by the reaction with acetic anhydride should be used.2.3Selection of the resin material and swelling propertiesPolystyrene (PS) is the most common core resin in solid-phasechemistry. Most polystyrene supports contain 1% or 2%divinylbenzene (DVB) as a cross linking agent, being insolublein all common solvents. Typically, these resins are small andspherical beads of two different sizes: 100–200 mesh (75–150microns) and 200–400 mesh (35–75 microns).Polyamines and polyethyleneglycols (PEG)-PS resins havebeen applied later to perform the synthesis of polar substratesand even in water solution.22 ChemMatrix resin, a totally PEGresin, has been recently developed for the synthesis of many longand complex peptides,23 with a higher loading capacity thanother PEG resins (from 0.2–0.3 mmol g 1 to 0.6–0.7 mmol g 1)and allowing the use of almost any kind of solvents, even water.The swelling of the polystyrene material in organic solventsis an important issue.10,11 This phenomenon has a very highin uence in the diffusion and accessibility of the reagents intothe core of the polymer and therefore to the synthetic efficiency.Thus, the typical swelling factor for a 1% DVB cross-linked PSresin goes from 5–6 times in THF, toluene, CH2Cl2, or dioxane, 4times in DMF, 2 times in EtOH or CH3CN, 1.6 times in MeOH upto no swelling in water.24 These swelling factors depend on thesort of resin.25 For example a resin with 2% DVB cross-linkingThis journal is The Royal Society of Chemistry 2014
View Article OnlinePublished on 18 July 2014. Downloaded by Secretaria General Adjunta de Informatica (SGAI) on 7/31/2019 8:22:53 AM.Reviewswells only 2 to 4 times its original volume in CH2Cl2 (Fig. 3A).The addition of Et2O to a CH2Cl2-swollen resin causes thecontraction of the beads size in more than 2 times (Fig. 3B).25 Anincubation of the resin in the corresponding solvent for 1 hstirring slowly is enough to complete the swelling.From a classic peptide synthesis, Merri eld observed thatthe swelling of the unloaded resin was higher in CH2Cl2 than inDMF. However, the swelling factor gradually decreased in thissolvent as the peptide content increased, and being increased inDMF (Fig. 3C).24 However, CH2Cl2 is not compatible withpiperidine, which is used to remove the Fmoc group, because ofthe formation of piperidine hydrochloride, and it is not a goodsolvent for Fmoc amino acids and some coupling reagents.Therefore, in a peptide synthesis using a PS resin, CH2Cl2 isnormally the major solvent at the beginning and sometimes ascleavage solvent. Mixtures of DMF–CH2Cl2 are used in SPPSusing PS resin in the rst coupling whereas DMF is the mainsolvent a er several couplings (Fig. 3C). However, PEG resinsswell properly in a large variety of solvent, even acetonitrile.26RSC AdvancesPS-resins chemically functionalized by methylchloride(Merri eld resin), bromide, hydroxy methyl (HM), aminomethyl(AM), carboxylic acid or aldehyde are commercially available.Using the Merri eld resin, the rst amino acid is directlycoupled without previous activation, although the cleavecondition involves the hazards and inconvenience of workingwith HF, high pressure gas manifold, the inability to useglassware, and a highly poisonous reagent.10 HM and AM resinshave long been used in solid-phase peptide synthesis as a coreresin to which various linkers could be attached through astable amide bond.2.4Linkers for SPPSLinkers are the chemical units used to attach an amino acid to aresin bead for performing molecule growing in solid phase. Thenature of the linker determines the chemistry to be used, andespecially the conditions under the products can be cleavedfrom the resin. A wide variety of linkers for polystyrene coreresin have been developed in the last years.15 Table 1 summarizes some of the most popular resins for SPPS (commerciallyavailable) with different linkers with the particular cleavageconditions and the nal C-terminal functionalization obtainedin the synthesized peptide in each case.17,27–30 Most of them useacidic conditions for the cleavage, yielding peptides containinga carboxylic acid (e.g. Wang resin) or an amide (e.g. MBHA resin)at the C-terminus. Hydrazides or sulfonamides attach to theresin allow for the release of the peptide equipped with adifferent functional group by orthogonal mild cleavage conditions (Table 1). Albericio and coworkers have recently developeda new resin for SPPS with a better performance compared withthe traditional Wang resin.31 This is based on the use of a linkerwith two features: methoxy groups as the only activating groupsof the phenyl ring and a copper(I)-catalyzed click chemistryreaction to anchor it to the solid support.312.5Orthogonal strategy of protecting groupsThe correct choice of the protecting groups of the side-residuesin the amino acids represents a crucial step before starting thepeptide synthesis.32Acidic labile protecting groups are the most used in Fmocchemistry (basic labile). Actually, a repertoire of different protecting groups for each amino acid exists, and the deprotectionconditions depend on the speci c amino acid. Table 2 shows asummary of some of the most popular AA protecting groups andthe corresponding deprotection conditions.32For example, protecting groups stable in acidic and basicconditions such as Alloc in Lys or thiotBu in Cys show adifferent deprotecting mechanism, Pd catalysis or thioldisul de exchange respectively (Table 2).Fig. 3 Photographs of swelling effect on resin beads by the solvents.(A) (Left to right) The expansion of polystyrene beads 2% DVB crosslinked in CH2Cl2. (B) (Left to right) The contraction of pre-swollenpolystyrene beads in Et2O. This figure is reprinted with permission.25(C) Expansion of polystyrene beads 1% DVB crosslinked and peptide-Psresin showing relative dimensions before and after swelling (adaptedfrom ref. 24).This journal is The Royal Society of Chemistry 20142.6Activation of the carboxyl group of the amino acidsThe addition of a coupling reagent to activate the a-carboxylgroup of the amino acid is necessary for a rapid and quantitativeamide bond formation. Fig. 4 shows a list of the most usedactivating groups and activation mechanism.13RSC Adv., 2014, 4, 32658–32672 32661
View Article OnlineRSC AdvancesTable 1Different resins for SPPSResin namePublished on 18 July 2014. Downloaded by Secretaria General Adjunta de Informatica (SGAI) on 7/31/2019 8:22:53 AM.ReviewResin structureCleavage conditionsPeptide productWang resin90–95% TFA in CH2Cl2 1–2 hAcidRink acid resin1–5% TFA in CH2Cl25–15 min or 10% AcOHin CH2Cl2, 2 hAcidHMPB resin1% TFA in CH2Cl2 2–5 minAcid2-Chlorotrityl chloride resin1–5% TFA in CH2Cl2 1 minAcidSASRIN resin1% TFA in CH2Cl2 5–10 minAcidRink amide resin50% TFA in CH2Cl2 1 hAmideMBHA resinHF 0 C, 1 hAmideHydrazine resinCu(OAc)2, pyridine, acetic acid,nucleophile in CH2Cl2 or THFAcid, ester, amide1. ICH2CN/DIPEA/NMP, 24 h2. Nucleophile, DMAP, 24 hSulfonamide resinHMBA resinNaOH, N2H4, NH3 inMeOH 24 hROHLiBH4PEGA–BAL resinTFA–TFMSA (19 : 1)Dicyclohexylcarbodiimide (DCC) was rst used by Merri eld11 as the simplest and most popular reagents for carboxylactivation. The reaction was performed CH2Cl2 or DMF,11although dicyclohexylurea, a byproduct formed from DCC, isnearly insoluble in most organic solvents. Therefore, DCC isvery useful in solution phase reactions, but is not appropriate32662 RSC Adv., 2014, 4, 32658–32672Acid, ester, thioester,amide, etc.Acid, hydrazide, amideEsterAlcoholAcidfor reactions on resin. Diisopropylcarbodiimide (DIC) is usedinstead in solid phase synthesis since the urea byproductremains in solution. However, a partial racemization processhas been observed in this activation, because the O-acylisoureaformed is very reactive causing loss of chiral integrity of theamino acid. The addition of 1-hydroxybenzotriazole (HOBt) hasThis journal is The Royal Society of Chemistry 2014
View Article OnlineReviewTable 2RSC AdvancesProtecting groups in the aminoacids residuesPublished on 18 July 2014. Downloaded by Secretaria General Adjunta de Informatica (SGAI) on 7/31/2019 8:22:53 AM.Amino acidProtecting groupStructureDeprotection conditionsPmc90–95% TFA in CH2Cl2, 2–4 hPbf90% TFA in CH2Cl2/TIS, 1 h (longer times in multipleArg containing peptides)Asp/GluOtBu90% TFA in CH2Cl2, 30 minAsn/GlnTrt90% TFA in CH2Cl2, 30–60 minMtt1% TFA in CH2Cl2, 60 minTrt90% TFA in CH2Cl2, 30 minStBuTFMSAMttTrt15% TFA in CH2Cl2, 1 h90% TFA in CH2Cl2, 30 minBoc90% TFA in CH2Cl2, 30 minBocMtt90% TFA in CH2Cl2, 30 min1% TFA in CH2Cl2, 30 minAllocPd(PPh3)4 (5 mol%), PhSiH3, THF/CH3OH, 12 htBu90% TFA in CH2Cl2, 30 minTrtBocAlloc1% TFA in CH2Cl2, 2 h95% TFA in CH2Cl2, 1 hPd(PPh3)4 (5 mol%), methylanilin,DMSO–THF–0.5 M HCl (1 : 1 : 0.5), 8 h2% TFA in CH2Cl235% TFA in CH2Cl2Pd(PPh3)4 (5 mol%), PhSiH3, inimized this problem, because the corresponding benzotriazolyl ester is less reactive, more stable and less prone toracemization than the O-acylisourea.In this way, considering the problems using carbodiimidesduring the coupling, onium (aminium/uronium and phosphonium) salts were developed.12 The most widely used salts are N-This journal is The Royal Society of Chemistry ne]-N-methylmethanaminium hexa uorophosphate N-oxide (HBTU), um hexa uorophosphate (PyBOP) or no-1-ylmethylene}-N-methylmethan-aminium hexa uorophosphate (HATU), similar to HBTU but reacts faster withRSC Adv., 2014, 4, 32658–32672 32663
View Article OnlinePublished on 18 July 2014. Downloaded by Secretaria General Adjunta de Informatica (SGAI) on 7/31/2019 8:22:53 AM.RSC AdvancesReviewWhen the Fmoc deprotection is slow or incomplete, piperidine can be replaced by 1,8-diazabicyclo[5.4.0]undec-7-ene(DBU) (1% (v/v)) in DMF (2 30 s). DBU, a non nucleophilicbase, can improve the deprotection yield and thus the yield ofthe desired peptide. This base substitution can be also performed in the case of the presence of thioesters in the peptidesequence, such as the palmitoylated peptides.27Aspartamide side products formation, for example whenusing DBU, can be avoided using Fmoc-Asp(OMpe)-OH foramino acid coupling with the addition of 5% of formic acid tothe standard Fmoc cleavage agent solution.34Aggregation effects can be observed in some peptidesequences by chain elongation. The use of PEG-PS resin, DMFand some additives such as detergents (1% (v/v)) are some of thealternatives to reduce the problem. Particularly Ala, Ile, Met andLys are the most prone to aggregation, whereas Pro, Arg or Hishave low levels of aggregation.The protecting groups of amino acid residues must beremoved once the peptide sequence is nished. This part can beperformed before the cleavage, the last step, or at the same timeusing the conditions described in Table 2. Finally the peptidecleavage from the solid-support is performed according to theconditions requested for the corresponding linker (Table 1).3. Synthesis of tailor-made peptidesincluding post translationalmodifications on solid phase(A) Traditional coupling reagents for carboxylic acid activation.(B) HBTU/HOBt amino acid activation mechanism.Fig. 4less epimerization during coupling (Fig. 4).12 The coupling withthese salts must be combined with HOBt in the presence of abase, usually N,N-diisopropylethylamine (DIPEA). Tetramethyl uoroformamidinium hexa uorophosphate (TFFH) generatesacid uorides in situ which are very convenient for the incorporation of hindered amino acids.12,26 New coupling reagentsbased on the Oxyma scaffold, such as the uronium salt 1-cyano2-ethoxy-2-oxoethylidenaminooxy dimethylamino-morpholinocarbenium hexa uorophosphate (COMU),14,33 has been developed showing in some cases several advantages over classicbenzotriazole-based reagents.2.7Speci c considerations in SPPSParticular conditions of the coupling must be used, forexample, for cysteine due to the high degree of racemization.Then, the solvent for the coupling must be CH2Cl2–DMF (1 : 1),without pre-activation of the amino acid, using mainly collidine(trimethylpyridine, TMP) (5 equiv.), a weaker base than piperidine. These experimental conditions have been of particularinterest in the coupling of farnesylated cysteine to the resin as rst amino acid, such as in lipopeptide synthesis.27,2832664 RSC Adv., 2014, 4, 32658–32672Post-translational covalent modi cation phenomenon isessential for protein activation in Nature which is critical for theregulation of interactions with other proteins and small molecules.6,35 Between the different types of protein modi cations,35it can be emphasized: (i) lipidation (palmitoylation, myristoylation) and prenylation (farnesylation, geranylation),36 formembrane anchoring involved in vesicular transport and cellsignaling, growth, and differentiation, (ii) glycosylation, forextending protein half-life, targeting, and cell–cell interactions37and (iii) phosphorylation, for signal transduction38 (the mostrelevant and extended in Nature) (Fig. 5).Particularly, post-translational modi cations of polypeptidechains o en occur at multiple sites or in tandem cascades,crucial for biological function. For example, Abl tyrosine kinaseis found phosphorylated at eleven different sites (more than 4 107 possible phosphorylated isoforms for one protein)38 spreadover the different catalytic and regulatory domains of theprotein. The Ras protein superfamily, important proteinspresent in around 30% of all human cancers, suffer theconsecutive four different post-translational modi cations to bebiologically active.36 Mucins, epithelial surface proteins, areheavily glycosylated with complex linked O-oligosaccharidesinvolved in protection and control of the cell surface.39 However,the isolation of these modi ed proteins for biosynthesis usuallyreveals the presence of heterogeneous mixtures which make theinvestigations of their role in biological processes quite difficult.40 In particular fully lipid-modi ed parts of lipoproteins areo en not accessible in their completely lipidated form of gene-This journal is The Royal Society of Chemistry 2014
View Article OnlinePublished on 18 July 2014. Downloaded by Secretaria General Adjunta de Informatica (SGAI) on 7/31/2019 8:22:53 AM.ReviewFig. 5RSC AdvancesPost-translational protein modifications. Pal: palmitoyl, Myr: myristoyl, Far: farnesyl.technological methods. The pure homogenous modi edproteins need to be prepared by semisynthesis from suitablyfunctionalized synthetic peptides and expressed protein parts.Tailor-made peptides incorporating the speci c posttranslational modi cation/s, that embody the characteristicmodi ed amino acid sequences of their parent proteins, haveproven to be efficient reagents and chemical tools for chemicalbiological, biochemical, biophysical, structural-biological andcell-biological studies.For the efficient and rapid synthesis of these peptideconjugates, a exible solid-phase technique is required. At thisstage, a SPPS strategy that proceeds with preparative viableoverall yields must be selected, considering these additionalchemical groups incorporated on the amino acids, especially forpeptides including multiply post-translational changes thatrepresent the fully modi ed protein.Therefore, taking into account all the aspects about the solidphase synthesis described above, the selected technique wouldfeature very mild, preferably neutral conditions (e.g. the sensitivityof lipidated chains to acid or basic conditions), to allow for theintroduction of additional reporter groups (e.g., uorophores)and tags (e.g., for coupling to expressed proteins), and allow forthe release of the peptides from the solid support as carboxylicacids or esters (depending on the precise structure of theThis journal is The Royal Society of Chemistry 2014natural blueprint), or equipped with a different functionalgroup at the C terminus.3.1PhosphopeptidesNatural modi cation of proteins by phosphorylation occurs intyrosine, threonine and serine residues being the most relevantthe rst one.Phosphopeptides can be synthesized by two differentapproaches:41(i) Direct phosphorylation of the amino acid on resin (a eramino acid coupling or a er peptide is synthesized) e.g. usingdiarylphosphorochloridate or phosphoramidite reagents.Selectively orthogonal removable protecting groups on theserine, threonine or tyrosine residues to be phosphorylatedmust be used.(ii) The use of protected, phosphorylated amino acidbuilding blocks in the SPPS.The second option seems to be in general the best choice,avoiding side reactions such as the oxidation step of PIII (e.g.oxidation of cysteine, methionine or tryptophan residues) or theformation of H-phosphonates41 in the direct peptide phosphorylation (Fig. 6A).The feasibility of chemical synthesis of phosphorylatedpeptides by Fmoc-SPPS was greatly enhanced by the introduction of the monobenzyl protecting group for the phosphateRSC Adv., 2014, 4, 32658–32672 32665
View Article OnlinePublished on 18 July 2014. Downloaded by Secretaria General Adjunta de Informatica (SGAI) on 7/31/2019 8:22:53 AM.RSC AdvancesFig. 6 Problems in phosphopeptides synthesis. (A) I: phosphate, II:phosphonate. (B) N/O-acyl migration.group.41,42 This minimized b-elimination of the phosphategroup and an N-O-acyl migration at threonine residues(Fig. 6B)42 making Fmoc-based synthesis of phosphopeptidesthe preferred synthesis strategy. For example the use of FmocTyr(PO(OBzl)OH) avoids this problem and allows the improvement of both purity and yield of phosphopeptides. These OBzlgroups are cleaved in similar conditions to the cleavage in Wangresin (Table 1). PEGA resin has been successfully used in thesynthesis of this type of peptides. In particular, Jensen andcoworker synthesized a set of different phosphopeptides onpreloaded acryloylated O,O0 -bis(2-aminopropyl)polyethyleneglycol copolymer (PEGA) with the 4-formylphenoxyacetic acidderived backbone amide linker (BAL).43 This solid-supportedpeptides were used for pull-down and analysis of the affinitypro le of the integrin-linked kinase associated phosphatase(ILKAP), a member of the protein phosphatase 2C (PP2C)family.3.2Reviewb-elimination upon treatment with strong bases (Fig. 7).However, the former is stable to treatment with concentratedTFA at short times, especially when the hydroxyl groups of sugarare protected as esters. The latter is stable to bases, e.g. morpholine, piperidine, DBU, that are commonly used to removethe Fmoc protecting group for SPPS (Fig. 7).Thus, these labile characters limit the variety of protectinggroups that can be applied for glycopeptide synthesis.40,41Considering the assumptions discussed above, protectinggroups such as Trityl or Mtt (4-methytrityl) (deprotected by lowacidic conditions, see Table 2) would be the best choice foramino acid residues.The hydroxyl groups of the oligosaccharide moiety aregenerally protected by acetyl groups in order to avoid esteri cation of sugar hydroxyl groups during the elongation of thepeptide chain and stabilize the O-glycosidic linkages duringcleavage with TFA. The O-acetyl-groups can be removed bytreatment with dilute sodium methoxide in methanol, hydrazine hydrate or saturated ammonia in methanol. These reactionconditions, if carefully monitored, will not affect the glycopeptide structure and they are compatible to high concentrations ofTFA.45Thus for the SPPS of glycopeptides using the glycosylatedamino acid building blocks, Wang, Rink-amide, Sasrin or 2chlorotrityl chloride resin have been generally used.45These strategies of the solid phase, for example using thechlorotrityl resin, has been successfully applied in the synthesisof glycopeptides mimics of mucin sequences of epithelial tumorcells, one of the most promising tumor-associated antigens.44–47Very recently, a new strategy using hydrazine linker i
The solid-phase approach is the best alternative to synthesize these peptides rapidly and in high amounts. The key aspects that need to be considered when performing a peptide synthesis in solid phase of these molecules are discussed. 1. Introduction Peptide synthesis has been, for more than a century, a signi -