Transcription
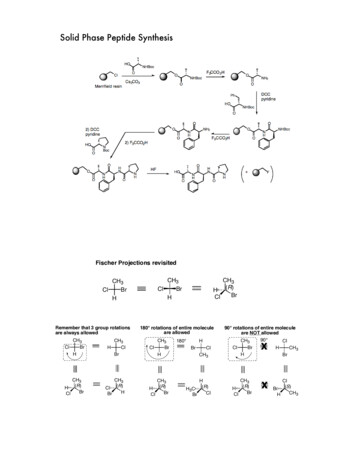
Solid Phase Peptide SynthesisFischer Projections revisitedCH3ClBrHRemember that 3 group rotationsare always allowedClCH3BrHCH3H ( R)BrClHCH3ClBrCH3Cl (R)HBrClCH3BrHCH3H ( R)BrCl180 rotations of entire moleculeare allowedClCH3BrHCH3H (R)BrCl180 HBrClCH3HH3C (R)ClBr90 rotations of entire moleculeare NOT allowed90 CH3ClClBrxHCH3H ( R)BrClHCH3BrxClBr (S)CH3H
mirror images are enantiomersClCH3BrHCH3H ( R)BrClBrCH3ClHCH3(S) HBrClidentification of meso compounds by a horizontal mirror planeMESO compoundsCH3HH(S )OHOH( R)CH3CH3CH3H(S)OHHOHHOHHOHHOHH(R)OHCH3HOHCH3mirror plane
a Helpful way to assign stereochemistry33CH3ClBrHCH32ClBr 1HCH3H ( R)Br 1Clnotice thatlowest priority group (H)is in the back424lowest priority group on bottom33CH34HBr 1ClCH3HBrClCH32(S)ClBr 1Hnotice thatlowest priority group (H)is in the front42lowest priority group on sideIf the lowest priority group is on the top or bottom: assign 'as normal'43CH32ClBr 1HHclockwise R3Cl 2FBrcounterclockwise S14BUT If the lowest priority group is on the SIDE: make the OPPOSITE ASSIGNMENT323CH3H4ClBrclockwise S(opposite of the 'normalassignment)4CH3HBr1F2counterclockwise R(opposite of the 'normalassignment)1CH3H(S)HOHOHH(R)CH3OHnot a stereocenter
Carbohydrates: CnH2nOHHOHHCHOOHHOHOHCH2OHsugarDextrorotatory D (R) configuration for sugarsD-glucose: an aldohexosealdehydeLevorotatory L (S)-configuration for sugarssix carbon(R)all of the natural sugars haveR configuration at this stereocenter.CH2OHOHOHHOHHOHCH2OHHHHD-fructose: a ketohexoseCHOOHOHOHCH2OHD-ribose: an aldopentoseOpen chain sugars can equilibrate with cyclic hemiacetalsCyclic hemiacetal HOHHOHHOHHOHOHOOK, so this is how they drew it back in the 1800’s
Haworth ProjectionsFor O54OHOHOH1H O23OH90 turnOH545OHOHOHOHO 14OH12OH3" anomerOOH23OHOH! anomerFor riboseCHOOHOHOHCH2OHHHHCHOHOHHOHHHOOHsquiggly linerepresents mixtureof ! and " anomersHOOOHOHOH90 turnConverting Haworth Projections to Chair ProjectionsFor 6523OHOH6O5OOH3OH! anomer41OHMutarotation of glucoseOHOHOHOOHOHcat H or OH–HOOHOOHHOHOHOHOHOO!D 112H2O!D 5223! anomerHOOHO5HOHO21HOaxialOH
Oligosaccharides and Polysaccharidesa ellulose : a glucose polymerHOmaltose (beer): a glucose dimerhighly linear strucrture: very effectivefor intrastrand H-bondinginsoluble: cell wallsstarch: two HO( 20%)‘soluble starch’HO!OOHOHO!!OOHO!OHO( 80%)‘insoluble starch’OHOOHOHOOligosaccharides and PolysaccharidesAmylose is highly helicalend viewamyloseOHOOHOHO!OHOHOOHOHO!O!Oetc
Some reactions of sugarsKiliani Fischer SynthesisseparableHOHHCHOHOHOHCH2OHHHOHH1) NaCN2) H2/PdBaSO43) H3O arabinosean aldopentoseCHOOHHOHOHCH2OHHOHOHH Mannosean aldohexoseglucosean aldohexoseH3O H2OHNHOHHOHOHCH2OHIntermediatefrom reductionstepa cyanohydrinSome reactions of sugarsWohl DegradationHHOHHCHOOHHOHOHCH2OH1) H2N-OH2) Ac2O; NaOMe3) H3O HOHHCHOHOHOHCH2 OHarabinosean aldopentoseglucosean aldohexoseH3O (retro cyanohydrin)H2N-OH– OAcHHHOHHN OHOHHOHOHCH2OHHAc 2OHAcOHHH N OAcOAcHOAcOAcCH2OAcNHAcOHHOAcHOAcOAcCH2 OAcNNaOMeHHOHHOHHOHOHCH2OH
Oxidation of sugarsHHOHHCHOOHHOHOHCH2OHHNO3xyloseThe Fischer ProofCO2HOHHOHOHCO2Hoptically OHCH2OHmesooptically inactivemirror plane
The Fischer HHNO 3HNO3HHOHoptically activeCO2HOHHOHCO2HHOHOHmesooptically activeCO2HHHOHCO2HThe Fischer ProofHHCO2HOHOHCO2HHNO 3HNO3HOHCO2HHOHCO2Hmesooptically 2Hoptically activeHNO 3HNO3HHOHCO2HOHHOHCO2HHOHOHmesooptically activeCO2HHHOHCO2H
The Fischer ProofIt is arabinoseHHCO2HOHOHCO2HHNO 3HNO3HOHCO2HHOHCO2Hmesooptically 2HHNO 3HNO3HHOHoptically activeCO2HOHHOHCO2HHOHOHmesooptically activeCO2HHHOHCO2HThe Fischer Proof: Fischer used this type of analysis for all of theSugars, starting from a good guess for glyceraldehydeIt is arabinoseHHCO2HOHOHCO2HHNO 3HNO3HOHCO2HHOHCO2Hmesooptically 2Hoptically activeHNO 3HNO3HHOHCO2HOHHOHCO2HHOHOHmesooptically activeCO2HHHOHCO2H
Solid Phase Peptide Synthesis CH 3 Cl Br H Fischer Projections revisited CH3 Cl Br H CH Cl H Br (R) Remember that 3 group rotations are always allowed CH3 ClBr H CH3 . Kiliani Fischer Synthesis CHO HOH HOH CH2OH HOH arabinose an aldopentose 1) NaCN separable HOH HOH CH2OH HOH HOH CHO glucose an aldohexose HOH HOH CH2OH HOH HOH CHO Mannose an .