Transcription
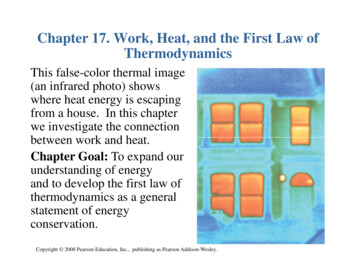
Chapter 17. Work, Heat, and the First Law ofThermodynamicsThis false-color thermal image(an infrared photo) showswhere heat energy is escapingfrom a house. In this chapterwe investigate the connectionbetween work and heat.Chapter Goal: To expand ourunderstanding of energyand to develop the first law ofthermodynamics as a generalstatement of energyconservation.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Chapter 17. Work, Heat, and the First Law ofThermodynamicsTopics: It’s All About Energy Work in Ideal-Gas Processes Heat The First Law of Thermodynamics Thermal Properties of Matter Calorimetry The Specific Heats of Gases Heat-Transfer MechanismsCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Chapter 17. Reading QuizzesCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
What quantities appear in the first lawof thermodynamics?A.B.C.D.E.force, mass, accelerationinertia, torque, angular momentumwork, heat, thermal energywork, heat, entropyenthalpy, entropy, heatCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
What quantities appear in the first lawof thermodynamics?A.B.C.D.E.force, mass, accelerationinertia, torque, angular momentumwork, heat, thermal energywork, heat, entropyenthalpy, entropy, heatCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
What was the original unit formeasuring heat?A. BTUB. WattC. JouleD. PascalE. CalorieCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
What was the original unit formeasuring heat?A. BTUB. WattC. JouleD. PascalE. CalorieCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
What is the name of an ideal-gasprocess in which no heat istransferred?A. IsochoricB. IsentropicC. IsothermalD. IsobaricE. AdiabaticCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
What is the name of an ideal-gasprocess in which no heat istransferred?A. IsochoricB. IsentropicC. IsothermalD. IsobaricE. AdiabaticCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Heat isA. the amount of thermal energy in an object.B. the energy that moves from a hotterobject to a colder object.C. a fluid-like substance that flows froma hotter object to a colder object.D. both A and B.E. both B and C.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Heat isA. the amount of thermal energy in an object.B. the energy that moves from a hotterobject to a colder object.C. a fluid-like substance that flows froma hotter object to a colder object.D. both A and B.E. both B and C.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
The thermal behavior of water ischaracterized by the value of itsA. heat density.B. heat constant.C. specific heat.D. thermal index.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
The thermal behavior of water ischaracterized by the value of itsA. heat density.B. heat constant.C. specific heat.D. thermal index.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Chapter 17. Basic Content and ExamplesCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Work in Ideal-Gas ProcessesConsider a gascylinder sealed atone end by amoveable piston.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Work in Ideal-Gas ProcessesIf we let the piston move in a slow quasi-static processfrom initial volume Vi to final volume Vf, the total workdone by the environment on the gas isor, graphicallyCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Problem-Solving Strategy: Work in Ideal-GasProcessesCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Problem-Solving Strategy: Work in Ideal-GasProcessesCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Problem-Solving Strategy: Work in Ideal-GasProcessesCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Problem-Solving Strategy: Work in Ideal-GasProcessesCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Work in Ideal-Gas ProcessesIn an isochoric process, when the volume does not change,no work is done.In an isobaric process, when pressure is a constant and thevolume changes by V Vf Vi, the work done during theprocess isIn an isothermal process, when temperature is a constant,the work done during the process isCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.2 The work of an isothermalcompressionQUESTION:Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.2 The work of an isothermalcompressionCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.2 The work of an isothermalcompressionCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.2 The work of an isothermalcompressionCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Heat, Temperature, and Thermal Energy Thermal energy Eth is an energy of the system due tothe motion of its atoms and molecules. Any system hasa thermal energy even if it is isolated and notinteracting with its environment. The units of Eth areJoules. Heat Q is energy transferred between the system andthe environment as they interact. The units of Q areJoules. Temperature T is a state variable that quantifiesthe “hotness” or “coldness” of a system. Atemperature difference is required in order for heat to betransferred between the system and the environment. Theunitsof T are degrees Celsius or Kelvin.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
The First Law of ThermodynamicsWork and heat are two ways of transfering energybetween a system and the environment, causing thesystem’s energy to change. If the system as a whole isat rest, so that the bulk mechanical energy due totranslational or rotational motion is zero, then theconservation of energy equation isCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Temperature Change and Specific HeatThe amount of energy that raises the temperature of 1 kg ofa substance by 1 K is called the specific heat of thatsubstance. The symbol for specific heat is c.If W 0, so no work is done by or on the system, then theheat needed to bring about a temperature change T isCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Temperature Change and Specific HeatCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.3 Quenching hot aluminum inethyl alcoholQUESTION:Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.3 Quenching hot aluminum inethyl alcoholCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.3 Quenching hot aluminum inethyl alcoholCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.3 Quenching hot aluminum inethyl alcoholCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Phase Change and Heat of TransformationA phase change is characterized by a change inthermal energy without a change in temperature.The amount of heat energy that causes 1 kg ofsubstance to undergo a phase change is called the heatof transformation of that substance.The symbol for heat of transformation is L.The heat required for the entire system of mass M toundergo a phase change isCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Phase Change and Heat of TransformationTwo specific heats of transformation are the heat offusion Lf, the heat of transformation between a solidand a liquid, and the heat of vaporization Lv, the heatof transformation between a liquid and a gas. The heatneeded for these phase changes isCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.4 Turning ice into steamQUESTION:Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.4 Turning ice into steamCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.4 Turning ice into steamCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.4 Turning ice into steamCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.4 Turning ice into steamCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
CalorimetrySuppose to systemsstart at differenttemperatures T1 andT2. Heat energy willnaturally betransferred from thehotter to the coldersystem until theyreach a commonfinal temperature Tf.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Problem-Solving Strategy: CalorimetryProblemsCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Problem-Solving Strategy: CalorimetryProblemsCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Problem-Solving Strategy: CalorimetryProblemsCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Problem-Solving Strategy: CalorimetryProblemsCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
The Specific Heats of GasesIt is useful to define two different versions of the specificheat of gases, one for constant-volume (isochoric)processes and one for constant-pressure (isobaric)processes. We will define these as molar specific heatsbecause we usually do gas calculations using moles insteadof mass. The quantity of heat needed to change thetemperature of n moles of gas by T iswhere CV is the molar specific heat at constant volumeand CP is the molar specific heat at constant pressure.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
The Specific Heats of GasesCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
ConductionCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
ConductionFor a material of cross-section area A and length L,spanning a temperature difference T TH – TC, therate of heat transfer iswhere k is the thermal conductivity, whichcharacterizes whether the material is a good conductorof heat or a poor conductor.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
ConductionCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.10 Keeping a freezer coldQUESTION:Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.10 Keeping a freezer coldCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.10 Keeping a freezer coldCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
EXAMPLE 17.10 Keeping a freezer coldCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
ConvectionAir is a poor conductor of heat, butthermal energy is easily transferredthrough air, water, and other fluidsbecause the air and water can flow. Apan of water on the stove is heated at thebottom. This heated water expands,becomes less dense than the water aboveit, and thus rises to the surface, whilecooler, denser water sinks to take itsplace. The same thing happens to air.This transfer of thermal energy by themotion of a fluid—the well-known ideathat “heat rises”—is called convection.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
RadiationAll objects emit energy in the form of radiation,electromagnetic waves generated by oscillating electriccharges in the atoms that form the object. If heat energy Qis radiated in a time interval t by an object with surfacearea A and absolute temperature T, the rate of heat transferis found to beThe parameter e is the emissivity of the surface, a measureof how effectively it radiates. The value of e ranges from 0to 1. σ is a constant, known as the Stefan-Boltzmannconstant, with the value σ 5.67 10–8 W/m2K4.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Chapter 17. Summary SlidesCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
General PrinciplesCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
General PrinciplesCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Important ConceptsCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Important ConceptsCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Important ConceptsCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Important ConceptsCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Summary of Basic Gas ProcessesCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Chapter 17. QuestionsCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
A gas cylinder and piston are covered withheavy insulation. The piston is pushed intothe cylinder, compressing the gas. In thisprocess, the gas temperatureA. decreases.B. increases.C. doesn’t change.D. There’s not sufficient information to tell.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
A gas cylinder and piston are covered withheavy insulation. The piston is pushed intothe cylinder, compressing the gas. In thisprocess, the gas temperatureA. decreases.B. increases.C. doesn’t change.D. There’s not sufficient information to tell.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Two process are shown that take an idealgas from state 1 to state 3. Compare thework done by process A to the work doneby process B.A. WA WBB. WA WBC. WA WB 0D. WA WB but neither is zeroCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Two process are shown that take an idealgas from state 1 to state 3. Compare thework done by process A to the work doneby process B.A. WA WBB. WA WBC. WA WB 0D. WA WB but neither is zeroCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Which of the following processes involveheat?A. The brakes in your car get hot when youstop.B. You push a rigid cylinder of gas across africtionless surface.C. A steel block is placed under a candle.D. You push a piston into a cylinder of gas,increasing the temperature of the gas.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Which of the following processes involveheat?A. The brakes in your car get hot when youstop.B. You push a rigid cylinder of gas across africtionless surface.C. A steel block is placed under a candle.D. You push a piston into a cylinder of gas,increasing the temperature of the gas.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Which first-law bar chartdescribes the process shownin the pV diagram?Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Which first-law bar chartdescribes the process shownin the pV diagram?Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Objects A and B are brought into close thermalcontact with each other, but they are well isolatedfrom their surroundings. Initially TA 0 C andTB 100 C. The specific heat of A is more thanthe specific heat of B. The two objects will soonreach a common final temperature Tf. The finaltemperature isA. Tf 50 C.B. Tf 50 C.C. Tf 50 C.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Objects A and B are brought into close thermalcontact with each other, but they are well isolatedfrom their surroundings. Initially TA 0 C andTB 100 C. The specific heat of A is more thanthe specific heat of B. The two objects will soonreach a common final temperature Tf. The finaltemperature isA. Tf 50 C.B. Tf 50 C.C. Tf 50 C.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
For the two processes shown, which ofthe following is true:A. QA QB.B. QA QB.C. QA QB.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
For the two processes shown, which ofthe following is true:A. QA QB.B. QA QB.C. QA QB.Copyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Suppose you are an astronaut in space,hard at work in your sealed spacesuit.The only way that you can transferexcess heat to the environment is byA. conductionB. radiationC. convectionD. evaporationCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Suppose you are an astronaut in space,hard at work in your sealed spacesuit.The only way that you can transferexcess heat to the environment is byA. conductionB. radiationC. convectionD. evaporationCopyright 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.
Title: Microsoft PowerPoint - Chapter17 [Compatibility Mode]