Transcription
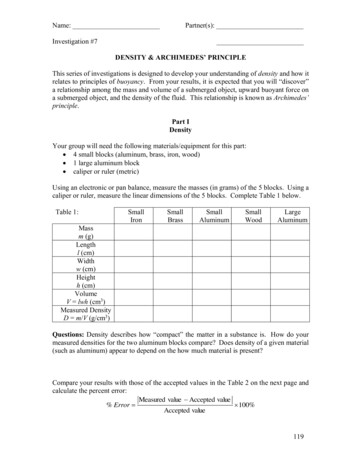
Name:Partner(s):Investigation #7DENSITY & ARCHIMEDES’ PRINCIPLEThis series of investigations is designed to develop your understanding of density and how itrelates to principles of buoyancy. From your results, it is expected that you will “discover”a relationship among the mass and volume of a submerged object, upward buoyant force ona submerged object, and the density of the fluid. This relationship is known as Archimedes’principle.Part IDensityYour group will need the following materials/equipment for this part: 4 small blocks (aluminum, brass, iron, wood) 1 large aluminum block caliper or ruler (metric)Using an electronic or pan balance, measure the masses (in grams) of the 5 blocks. Using acaliper or ruler, measure the linear dimensions of the 5 blocks. Complete Table 1 below.Table uminumMassm (g)Lengthl (cm)Widthw (cm)Heighth (cm)VolumeV lwh (cm3)Measured DensityD m/V (g/cm3)Questions: Density describes how “compact” the matter in a substance is. How do yourmeasured densities for the two aluminum blocks compare? Does density of a given material(such as aluminum) appear to depend on the how much material is present?Compare your results with those of the accepted values in the Table 2 on the next page andcalculate the percent error:Measured value Accepted value% Error 100%Accepted value119
Table 2:Measured Density(g/cm3)Accepted Density(g/cm3)IronBrassAluminumWood7.878.72.70 0.8% ErrorCheckpoint: Consult with your instructor before proceeding. Instructor’s OK:Part IIBuoyant ForceIn this section, you will explore volume displacement and how it relates to buoyancy.Your group will need the following additional materials/equipment for this part: Computer with LoggerPro software installed1 Dual-range force sensor (set on the 10-N scale)1 universal laboratory interface (ULI) box1 ring stand1 “spill-over” can, catch basin, and graduated cylinderProcedure1. Check that the ULI box is powered, properly connected to the computer and has theforce sensor plugged into CHANNEL 1.2. Set up the force analysis program on the computer by opening the Conceptual Physicsfolder on the computer desktop.3. Open the file Force Probe in that folder. When the file opens, a “Page Information”window should appear. Click “OK.” After a few seconds another window called“Sensor Confirmation” may appear. (If so, the confirmation should indicate that theforce sensor is plugged into Channel 1.) Click “Connect.” The Collect button at thetop of the screen should turn green indicating that the computer is ready to collect data.Otherwise, inform your instructor if there are persistent problems.4. Once you have successfully opened the proper file, the computer will display a graphof “Force vs. Time.” Note that the units for the force axis are Newtons and the unitsfor the time axis are seconds.5. Check that the force sensor is set on the 10-N scale. Place the force sensor on the ringstand so that the hook is downward. Attach a string to the sensor hook. Be sure thatthe sensor is hanging vertically.6. Make sure that you “zero” the force probe (while it is vertical and has nothing but thestring attached) by clicking the Zero button next to the Collect button.120
7. For each of the 5 blocks, measure the weight (inNewtons) in air using by hanging each blockindividually from the force sensor and taking themeasurement as shown in Fig.1. The totalmeasurement might take about 10 seconds,NO!OK.however you can stop after about 3 seconds. Besure that the material is hanging freely and issteady. The force measurement should appearas a horizontal line across the screen. If not, tryagain. To get the average weight over the timeinterval of the measurement, click the StatisticsFig. 1: Correct and incorrectbutton (designated by STAT) on the menu bar.orientations of the force probe.8. Take the mean value for measurement. Recordthe “Weight in Air” results in the Table 3 on thenext page.9. Fill the “spill-over” can so that it is “just on the verge” of spilling before you immerseany object. To set up this condition, place the can where you intend to make themeasurement and fill the can until liquid starts to emerge from the spout while usingthe catch basin to collect this excess fluid. When the water stops dripping, the can is“just on the verge” of spilling. Set aside the catch basin with the excess water.10. Start with one of the small metal blocks, say iron.Weigh the block while it is submerged in waterby carefully lowering the block into the spill-overcan as shown in Fig. 2. As you do this, use a drygraduated cylinder to collect the water that comesout of the spout as the block is immersed. (Takecare not to lose any of the displaced water.) Afterthe block is immersed, get a statistical averagefor the weight in water just as you did whileweighing the blocks in air. Be sure that the blockdoes not touch the side or bottom of the can whilemeasuring its weight. Record the “Weight inWater” of the block in Table 3. Record thedifference between the air and watermeasurements in the next column of the table.Using your data from Part I, reenter the volume Fig. 2: Weighing objects in water.of the block in the next column.Question: You know you can use the graduated cylinder to measure the volume (in cm3) ofthe water displaced by the blocks. You also need to measure the weight (in N) of thedisplaced water. Explain how you will do this.121
Checkpoint: Consult with your instructor before proceeding. Instructor’s OK:11. Record the volume and weight of the displaced water in the last two columns of Table 3below.12. Refill the “spill-over” can so that it is again “just on the verge” of spilling. Repeat themeasurements you just did for the four remaining blocks.Table 3MaterialWeight inAirWa (N)Weight in WeightVolume of Volume ofWater Difference*Object DisplacedWw(N) Wa - Ww (N)(cm3) Water (cm3)Weight ofDisplacedWater (N)IronBrassSmallAluminumWoodLargeAluminum*Note: The “weight difference” between the air and water measurements is equal to thebuoyant force that the fluid exerts on the immersed object.Question: Which (if any) of the following do all of the small blocks appear to have incommon: weight, mass, or volume?Question: How does the buoyant force (that is, the weight difference) for each block sampleroughly compare to the weight of the displaced fluid?Question: How does the volume of each block compare with the volume of displaced water?Was this true for all 5 blocks? If not, which blocks (if any) behaved differently and why doyou think this is so?Checkpoint: Consult with your instructor before proceeding. Instructor’s OK:122
Part IIIOdd Shapes and Different FluidsQuestion: Measuring the mass of an irregularly shaped object is easy enough, but how canyou measure its volume if the length, width and height cannot be measured accurately witha ruler or caliper due to the peculiar shape?Your group will need the following additional materials/equipment for this part: a piece of granite a piece of clay salt water solutionProcedure1. Perform the same measurements you did in Part II for two additional materials, graniteand clay. Record the results in Table 4 below.Table 4MaterialWeight inAirWa (N)Weight inWaterWw(N)BuoyantForceWa - Ww (N)Volume ofDisplacedWater (cm3)Weight ofDisplacedWater (N)GraniteClay2. Repeat Step 1 using the salt water, one of the small metal cubes of your choice, and thepiece of granite. Complete Table 5 below.Table 5MaterialWeight in AirWa (N)Weight inFluidWw(N)BuoyantForceWa - Ww (N)Volume ofDisplacedFluid (cm3)Weight ofDisplacedFluid (N)Cubein Salt WaterGranitein Salt WaterClayin Salt Water123
Question: Archimedes’ principle states: an object submersed in a liquid is buoyed up by aforce that has a magnitude equal to the weight of fluid displaced by the object. For all ofyour measurements in Part II and Part III, do your results generally confirm or refute thisstatement? (Hint: See Tables 3-5. Again, the magnitude of the buoyant force is equal to theweight difference between the air and water measurements.)Checkpoint: Consult with your instructor before proceeding. Instructor’s OK:Part IVSinking and FloatingFor your final set of measurements, you will determine how the relative densities of an objectand a fluid determine whether a particular object will sink or float in a particular fluid.Your group will need the following additional materials/equipment for this part: 1 polyvinyl chloride (PVC) specimen (white plastic) 1 acetate specimen (clear plastic) 1 Styrofoam brick 1 BB 3 beakers of fluid: 1 water, 1 alcohol (ethyl or isopropyl), 1 salt water 1 tubPrediction: How does the density of an object that sinks in a fluid compare to the density ofthat fluid? How does the density of an object that floats in a fluid compare to the density ofthat fluid?Question: Devise a method to measure the density of a fluid and explain it below.Checkpoint: Consult with your instructor before proceeding. Instructor’s OK:124
Procedure1. Measure the density of each of the three liquids (water, alcohol and salt water). Calculatethe percent error and complete Table 6 below.Table 6:Measured Density(g/cm3)Actual Density(g/cm3)WaterAlcoholSalt Water1.000.821.0 1.2% Error—2. Drop the two plastic specimens into a small beaker of water and observe whether eithersinks or floats. Remove the cubes and dry them. Repeat this observation for alcohol andthe saltwater solution. List your observations in Table 7 below.Table 7:WaterAlcoholSalt WaterPVC (White)Acetate (Clear)3. Measure the masses of the PVC and acetate specimens using the electronic pan balanceand record the results in Table 8 below.4. Measure the volumes of the PVC and acetate specimens using the calipers and record theresults in Table 8. (Look up the formula for the volume of the shape of the specimen ifyou do not remember.)5. Calculate the densities of the PVC and acetate and record the results in Table 8.Table 8:Mass (g)Volume (cm3)Density (g/cm3)PVC (White)Acetate (Clear)125
Question: Compare your calculated density for the PVC to the actual densities of the threefluids given in Table 6. Circle your results.DPVC DWaterDPVC DWaterDPVC DWaterDPVC DAlcoholDPVC DAlcoholDPVC DAlcoholDPVC DSalt WaterDPVC DSalt WaterDPVC DSalt WaterQuestion: Compare your calculated density for the acetate to the actual densities of the threefluids. Circle your results.DAcetate DWaterDAcetate DWaterDAcetate DWaterDAcetate DAlcoholDAcetate DAlcoholDAcetate DAlcoholDAcetate DSalt WaterDAcetate DSalt WaterDAcetate DSalt WaterQuestion: Are your measured densities for the PVC and acetate consistent with yourobservations of those two materials behaved in the three fluids? In other words, do yourresults match the prediction you made on page 124? If not, why not?6. In your Pre-lab, you predicted whether a large Styrofoam brick and a small metal BBwould sink or float when placed in water. Now test your predictions with the materialsprovided. Record your observations.The Styrofoam brickThe metal BBin water.in water.Question: Based on these results, what can be said regarding the density of Styrofoam andthe density of the metal in comparison to the density of water?Checkout: Consult with your instructor before leaving the lab. Instructor’s OK:126
Computer with LoggerPro software installed 1 Dual-range force sensor (set on the 10-N scale) 1 universal laboratory interface (ULI) box 1 ring stand 1 "spill-over" can, catch basin, and graduated cylinder Procedure 1. Check that the ULI box is powered, properly connected to the computer and has the











