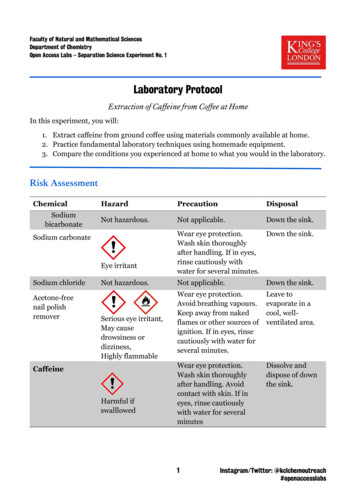
Transcription
E x p e r i m e n t1 8Isolation of Caffeine from TeaObjectives To extract caffeine from tea To purify the caffeine via recrystallization To monitor the extraction and purification steps via Thin Layer Chromatography(TLC)In the Lab Students work in pairsAfter Lab Complete the lab report on Chem21Labs.comWaste Place the used potassium carbonate in the waste container in the hood. Place aqueous solutions that remain at the end of lab in the waste container labeledAqueous Waste located in the Instructor’s hood. Place organic solutions that remain at the end of lab in the waste container labeledOrganic Waste.Safety Students must wear goggles for this experiment. Gloves are available.
Caffeine belongs to a class of organic compounds called alkaloids. Alkaloids are naturallyoccurring compounds that contain nitrogen – a structural feature that gives these compoundsbasic / alkaline properties. Common alkaloids include caffeine (stimulant), nicotine, andmorphine neOther common alkaloids include cocaine (stimulant), atropine (spasmolysis agent),vincamine (vasodilator), quinine (anti-malarial), ephedrine (anti-asthma), and codeine(analgesic). Note the “ine” ending for the alkaloids.Caffeine is commonly found in coffee, tea, soft drinks, chocolate, and "stay-awake" pillssuch as NoDoz (see below). Tea leaves contain cellulose, chlorophyll, tannins, and caffeine( 4%). Caffeine stimulates the heart, central nervous system, and the respiratory system. Itis a diuretic and has the effect of delaying fatigue. One can develop both a tolerance and adependence on caffeine. The dependence is real, and a heavy user ( 5 cups of coffee perday) will experience lethargy, headache, and perhaps nausea after about 18 hours of non-use.Caffeine SourceCaffeine AmountCoffeeCoffee, decaffeinatedTeaCocoaMilk chocolateBaking chocolateCoca-ColaAnacinExcedrin, extra strengthDexatrimNo-Doz80 to 125 mg per cup2 to 4 mg per cup30 to 75 mg per cup5 to 40 mg per cup6 mg per oz35 mg per oz46 mg per 12 oz32 mg per tablet65 mg per tablet200 mg per tablet100 mg per tabletPure Caffeine has a melting point of 235 oC - 238 oC, and a molecular weight of 194.19g/mol. It is very soluble in Methylene Chloride and partially soluble in water. The isolationof Caffeine from tea appears rather simple, while in fact it utilizes a number of rather
advanced chemical processes. To isolate the Caffeine in a sample of tea, it is necessary tochemically separate the Caffeine from the rest of the tea (mainly cellulose). The procedureused around the world to make a cup of tea will be used in the lab today. The tea is“steeped" in very hot water for about 10 minutes – this extracts most of the caffeine. Thereis no advantage to boiling the tea leaves with water for 10 minutes. Not only is caffeinemore soluble in hot water, but hot water is a critical component in that it causes the tea leavesto swell releasing the caffeine trapped in its cells.The resulting brown tea solution is then extracted with dichloromethane, dissolving primarilycaffeine and leaving the other water-soluble substances in the aqueous layer. In fact, theextraction is performed three times with fresh Methylene Chloride since multiple extractionsremove more Caffeine than a single extraction with the same total amount of solvent.Evaporation of the Methylene Chloride leaves behind crude caffeine, which onrecrystallization yields a relatively pure product.The solubility of caffeine in water is 2.2 mg/mL at 25 C, 180 mg/mL at 80 C, and 670mg/mL at 100 C. It is quite soluble in dichloromethane, the solvent used in this experimentto extract the caffeine from water.Table of Physical ConstantsChemical 4194.19Methylene Chloride(Dichloromethane)CH2Cl284.93Calcium oilingPointDensitynD40 C1.3251.424056 C.7911.3590.6561.3760Petroleum Ether(low-boiling)30 - 60 CPetroleum Ether(high-boiling)[Ligroin]60 - 80 CTable 120
Ex p e r i m e n t1 8Procedure:1. If the Instructor has prepared the tea,go to Step 9. Enter 8.0 g for the mass oftea and obtain 50.0 mL of the prepared tea.2. Place 50 mL hot water and 2.5 gramscalcium carbonate in a 250 mL beaker.3. Heat the beaker on a hot plate (setting10) until the water is boiling.4. Turn the heat off and place 3 tea bagsof black tea in the hot water.5. Let the tea “steep” in the hot solutionfor 10 minutes – leave the beaker on thehot plate during this time.6. Remove the tea bags from the solutionand squeeze all the liquid from them.7. Vacuum filter the tea solution througha pad of filter aid (Celite) to remove theCalcium carbonate.8. Place the filtrate in a beaker in an icebath for 10 minutes.9. Transfer the filtrate (or 50 mL ifalready prepared) to a separatory funneland add 25 mL of methylene chloride.10. Shake vigorously for 3 seconds andallow the layers to separate for 10 minutes.11. Remove the lower organic layer(s)(the two layers below the dark brownaqueous layer) and place it in a 250 mLErlenmeyer flask.12. Add 25 mL of fresh methylenechloride to the aqueous layer remaining inthe separatory funnel and shake vigorouslyfor 10 seconds and allow the layers toseparate for 5 minutes.13. Remove the lower organic layer andplace it in the Erlenmeyer flask containingthe first 25 mL of methylene chloride.14. Repeat Steps 12 and 13 – this willmake a total of 75 mL of methylenechloride.15. Rinse the separatory funnel with water(don’t dry it) and place the 75 mL ofmethylene chloride back into the funnel.Allow the lower organic layer to slowlypass through potassium carbonate granules(the instructor will give you the K2CO3when you are ready) that have beenplaced in the fluted filter paper in theapparatus shown. The glass funnel andErlenmeyer flask must be dry. If waterdroplets are observed in the Erlenmeyerflask after filtering, add 1 g K2CO3 tothe liquid, swirl vigorously and pass thesolution through a fluted filter paper into adry Erlenmeyer flask.16. Save 0.5 mL of this liquid in a cleantest tube (label Step 15).17. Place the rest of the organic layer intoa 250 mL RBF (24/40 ground glass joint)and remove the CH2Cl2 via rotaryevaporation (see apparatus below).18. Stop the evaporation when 5 mLremain (if the solvent completelyRemove StopperStopcockOpenDraining Isolation of Caffeine from TeaStopcockClosedSeparating418
Ex p e r i m e n t1 8from the solution. If caffeine does notprecipitate, heat the solution on a hot plateto remove 0.25 mL acetone and add 10more drops of low-boiling petroleumether. Keep removing 0.25 mL of acetoneand adding the petroleum ether untilcrystals form.25. Cool the crystals in an ice / water bathfor 5 minutes. Also cool 5 mL of lowboiling petroleum ether.26. Remove the “mother liquor” (theliquid that remains) from the crystals bysuction using a disposable pipette – pressthe tip of the pipette on the bottom of thebeaker and withdraw the liquid into thepipette taking care not to remove any ofthe caffeine crystals. If no liquid ispresent, go to Step 28.27. Save this liquid in a clean, dry, labeledtest tube (label Step 26).28. Wash the caffeine with 5 mL coldpetroleum ether and remove this solventwith a transfer pipette as described in Step26 – add this solvent to the test tube inStep 27.29. Place a tiny amount (a speck) of yourcaffeine crystals in a clean test tube (labelStep 29).30. Place the rest of your crystals in thedrying oven until you have completed theTLC analysis.CondenserYourCompoundDistillate Isolation of Caffeine from TeaWater Bathevaporates or you have 5 mL left, add 5 mL CH2Cl2 to dissolve the caffeine).19. Use a pipette to transfer the caffeine /CH2Cl2 solution to a 50mL beaker.Distillate20. Rinse the RBF several times withBathdistilled methylene chloride (don’t usemore than 10 mL total) and add theserinses to the 50mL beaker.21. On a hot plate in the hood evaporatethe CH2Cl2 solution to almost dryness(leave 1 mL). Swirl the solutioncontinually during evaporation, otherwisespattering will occur and your product willbe lost.22. The heat remaining in the beakershould evaporate off the remaining CH2Cl2leaving only solid caffeine in the beaker.If not, heat the beaker to remove anyremaining CH2Cl2.23. Add just enough acetone (0.5 – 2.0mL) to the impure caffeine to completelydissolve it when the acetone is boiling.24. Immediately remove the heat and add10 drops low-boiling petroleum ether(primarily pentane) to precipitate caffeineThin Layer Chromatography31. You should have three labeled testtubes containing caffeine at various puritylevels. At the front of the class is anothertest tube containing pure caffeine from thestockroom. Spot these four solutions on aThin Layer Chromatography (TLC) plateand develop the plate with 50% CH3OH /H2O. Place a line 1 cm from the top of theplate and remove the plate from the518
Ex p e r i m e n t1 818 Isolation of Caffeine from TeaLab Reportsolvent when the solvent just touches thisline. Use UV light to visualize your spots.Determine the Rf value of your caffeine.32. Weigh the product and obtain itsmelting point.33. Place your product in a labeled vialand turn it in to your instructor.34. Calculate the percent recovery fromthe weight of tea used.Once you have turned in your InstructorData Sheet, lab attendance will be enteredand you will have access to enter your labdata online and begin the lab submissionprocess. Enter you lab data before exitingthe lab - enter your data accurately toavoid penalty. The lab program will takeyou in order to each calculation. Mouseover the orange “TOL” link to see thepoints and tolerances for each calculation.Interpreting the DataThe Rf value for caffeine must be calculated. Rfstands for “ratio of fronts” and is characteristicfor any given compound on the same stationaryphase using the same mobile phase. In otherwords, you and any other person in the lab todayshould calculate the same Rf value for caffeinesince you are both using the same type ofchromatography medium, the same developingsolvent and essentially the same experimentalconditions.Rf D istan ce fro m start to cen ter o f s u b stan ce sp o ttedD istan ce fro m start to so lven t fro n tLab 18Isolation of Caffeine from TeaName:Mass:6g
Isolation of Caffeine from TeaStudent Data SheetWeight of Tea-gWeight of recrystallized CaffeinegMelting Point Range of Recrystallized CaffeineºCResults from Thin Layer Chromatography: “Reproduce”your TLC plate on the rectangle provided.Spots Name:Partner:Isolation of Caffeine from TeaInstructor Data SheetWeight of Tea-gWeight of recrystallized CaffeinegMelting Point Range of Recrystallized CaffeineºCResults from Thin Layer Chromatography: “Reproduce”your TLC plate on the rectangle provided.7Spots
To isolate the Caffeine in a sample of tea, it is necessary to chemically separate the Caffeine from the rest of the tea (mainly cellulose). The procedure used around the world to make a cup of tea will be used in the lab today. The tea is “steeped" in very hot water for about 10 minutes –