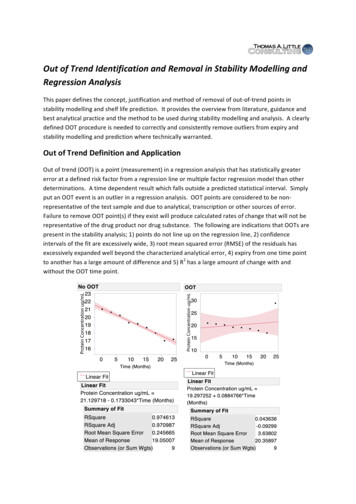
Transcription
Christopher A. Rhodes, PhD, President, CEO, FounderThe Pharmaceutical Technology SpecialistsExenatide and Its Life CycleEuroTIDES, 08 NOV 2017www.drugdeliveryexperts.com
Achieving Target Product Profile Requires A DeepUnderstanding of Active, Formulation, DeviceLeveraging a deep understanding of molecular properties, formulation, and deviceIntegrating delivery system R&D project into your development programOptimizing target product profile to enhance value propositionDiscovery Support Lead molecule profilingClinical candidate evaluationBiologic half-life extensionDrug Product DevelopmentDevice Development Formulation designDrug product developmentAnalytical methodsDevice identificationIntegration with formulationDevelopment and selection2
Market Preference for Non-invasive DeliveryInjectionOraldevice ---Transdermal --- patchNasalBuccalSublingualOnce per day BID or TID3
Injection Frequency PreferencesDecreasing Injection / Administration FrequencyMultipleDaily InjDailyInjectionProduct Profile Parameters Complexity of product handlingReady-to-use productNeedle size for injection (viscosity)Injection force (viscosity)Pain on injection (volume)Duration of injection (volume)In-Use stability yInjection6 to 12Month InjPatient Self-Injection Common:Product Profile More CriticalPotential for Office Administered Product:Good Product Profile Not Critical4
Commercially Approved Delivery Technologies forSystemic Delivery of PeptidesInjection SystemsImplantablesNon-InvasiveLHRHPulmonaryXTEN, ELP,PAS conjugatesRiskPEGylation, AcylationMicrospheresPenInjectorsVial andSyringeDulaglutide, albiglutideLIRA, Semaglutide,OmontysLHRH, sandostatin LAR,BydureonCharacteristics: Moderate to High BA Acceptable Variability Continuous ExposureRewardRiskFc and Albumin fusionOral (Systemic)Pulmozyme, InsulinMicroneedlePTH, glucagon phase 3NasalDDAVP, sCT, Buserelin, Nafarelin, Oxytocin Commercialized Products Products in DevelopmentCharacteristics: Low Dose Low BA Variability Pulsatile ExposureReward5
Importance of Target Product ProfileMost Parameters Affect the User ExperienceCriteriaSuggested for ConsiderationRoute of AdministrationSubcutaneous, Intravenous, IntramuscularNon-invasive (Nasal, microneedle)Dose Frequency andPharmacokineticsDaily or multiple daily injection (with native PK profile)Weekly, Monthly, Quarterly (with continuous exposure)Projected DoseProjected human, animal, toxicity doses (drives concentration in dosage form)Dose Volume 1mL for subcutaneous injection (also drives concentration in dosage form)Ease of Use and HandlingEasily injected through a 26G or smaller needleMinimal handling by care giver (simple reconstitution)Device and ContainerClosure SystemVial and syringe, pre-filled syringe, dual-chamber syringe, cartridgeMulti-use pen, or auto-injectorStability In-use25oC, 1 week to 1 monthStability for Long TermStorage2-8oC, minimum 24 months6
Sustained Release Formulation jectionMonthlyInjectionIncreasing Drug PotencySuspensionAtrigelLiposomeIn Situ Gel-Forming SystemMicrosphereNon-Aqueous Solution/SuspensionImplant7
Conjugate Approaches for Half-Life tionIncreasing Drug PotencyAcylation(albumin binder)CarbohydrateanaloguesHESylation, GlycosylationPoly AminoAcid FusionsPEGylationXTEN, ELP, PASylationVarious including reversible PEGAlbumin or Fc Fusion(FcRn recycling)8
Exenatide Properties – A Delivery Scientists DreamHighly potent drug – 10 to 20 micrograms per dayHighly water soluble peptides – 100s mg/mlGood stability in aqueous solutionGood metabolic stabilityHalf-life of 1 to 2 hours in humansChoices for delivery system are virtually unlimitedYet, mistakes can be (and were) made9
Drug Product Profile Example: ExenatideByetta (exenatide injection) Launched by Amylin and Eli Lilly Partnership (now owned by Astra Zeneca) Discovered by John Eng (VA Hospital) 1996Exenatide Drug Substance 39 amino acid peptideContainer Closure System 1.2 & 2.4 mL cartridge for pen 0.25 mg/mL strengthDisposable Pen-injector 5 mcg or 10 mcg per injection Storage : 2 year shelf-life In-use: 30 day period at RT10
GLP-1s Move to Maximize Continuous ExposureByetta (exenatide)Half-life 1-2 hrsHbA1CReduction-0.9%Liraglutide (Victoza)Half-life 13 hrs-1.5%-1.2%Last InjectionBydureon(exenatideMS)Follow-Up PeriodActive Treatment Period550Plasma Exenatide (pg/mL)500Kothare P A et al. J Clin 50US 2005EU 20060EU 2009US 20100481216Time (wk)2024Kim D et al. Dia Care2007;30:1487-1493EU 2011US 20121128
Microspheres to Achieve Continuous ExenatideExenatide Weekly PLGA Microspheres (SEM)License PLGA Technology from Alkermes (2000)(Neutropin Depot) was precedent for work12
Exenatide Microsphere Manufacture and QCExenatide MS Release and Polymer DegradationPolymer Type, Formulationand ProcessControls Release ProfileAnd PharmacokineticsExenatide MS Particle Size DistributionParticle Size isControlled byProcess andDictates Deviceand Needle Guage13
Exenatide Microsphere (Bydureon) Life Cycle Bydureon is an exenatide microsphere formulation Vial and syringe, pen, suspension in auto-injectorBydureon (EU 2011 US 2012)Once weekly SC injection2 mg per week doseVial and syringe presentationdiscontinued Jan 2016 with pen launchBydureon Pen (US 2014)Once weekly SC injection2 mg per week doseBydureon Bcise (US 2017)Once weekly SC MS suspension2 mg per week dose14
Importance of Bydureon Bcise ApprovalProduct Precedent for Microsphere Based Products Bcise is a MS suspension in Miglyol (MCT)Single-use auto-injector (‘3 step’)First microsphere in ready-to-use injectable suspension productNeedle sheath and no needle handling or observationStore in refrigerator laying flatMay be stored at room temperature for 4 weeks prior to useSubstantial product improvement for diabetic patients15
Dramatically Simplified Instructions-for-UseShake the autoinjector hard for at least 15seconds until the medicine is well mixedMedicine must be fully mixed before unlocking.Unlock device and firmly unscrew orange capPush the autoinjector against the skin and16hold it there for 15 seconds to get full dose
Bydureon Timeline For Development20051996John EngVA-AmylinNCE LicenseByettaFDAApprovalExendin-4 R&D2000AlkermesAmylinMS LIcense2012BydureonFDAApprovalExen MS R&D2003AmylinEli LillyAlliance2009AmylinEli LillyMS PlantAmylinDeviceGroup2014MS DualBydureonChamber PenFDAApproval20172008AmylinProductDevelopmentMS Suspension R&DBydureonBcise FDA17Approval
Bydureon: Single Dose PK Profile (10 to 12 weeks)SD PK Dose Selection Study 2.5 mg, 6 mg, 7 mg, 10 mgDose Selection Study 0.8 mg and 2 mg exenatide Initial release in first day subject of significant formulation and clinical work Target product profile was once per month injection – could not be achieved due to initial release 300 pg/ml was achievable with low initial release by weekly injection of the same formulation18
Exenatide Delivery Opportunities Evaluated withinAmylin Lilly Alliance (2000 to 2011) Nasal formulation taken into clinicTransdermal microporation taken into clinicPulmonary dry powder evaluated in preclinical workOral delivery evaluated in preclinical workAll of these formulations suffered from PK issues Low bioavailability, variability, shorter exposure times than SC injection19
Nasal Target Product Profile: Aqueous solution formulation Simple manufacturing process Commercially available devices Nasal peptide products in market BID or TID administrationPlasma Exenatide (pg/mL)Nasal Exenatide Human Data (Nastech Formulation)600 ug IN5 ug SC10 ug SC(previous study)4003002001000060120180240300360420480Time (min)Opportunity Abandoned - un-attractive from marketing perspective 3 or 4X Nasal Spray required to achieve AUC equivalent to SC Injection (and clinical effect)20
Transdermal Microporation Human Data (Altea)Transdermal Target Product Profile: Simple bandaid-like product administered w device No pain on administration Continuous 24 hour exposure (Bydureon-like) Once per day administration (twice as fall back)Opportunity Abandoned - due to significant investment requied (device, patch, manufacturing) Once per day 24 hour continuous exposure nearly achieved21
Exenatide Lessons Byetta was launched in a good pen, but, with a refrigeration pack Challenges of microsphere sustained release formulation not well understood Importance of device was recognized too lateBydureon dual chamber pen was difficult and took too long Initial interest in a once monthly productWeekly product was a compromise due to initial release from particlesBydureon was launched in a vial and syringe CMC post-approval supplement required to get RT for 30 daysAt launch, inferior to other weekly GLP-1 products on the marketBydureon MS suspension could have been completed earlier ( !)Decision to build MS plant instead of working with CMOs ( )Singular focus on MS investment prevented other meaningful approaches22
Exenatide Analogues with Clinical Data Lixisenatide (Zealand technology)Hanmi exendin-4 analogues with Fc conjugate(Sanofi)Versartis XTEN exenatidePhaseBio ELP exenatideMultiple programs in clinical development for analoguesbased on exenatide23
Numerous Approaches for Long-ActingFormulations and AnaloguesDrug Design, Development and Therapy2013:7 963–97024
GLP-1 AGONIST MOLECULAR ENGINEERINGTwice Daily InjectionOnce Daily InjectionEx-4 plus poly Lys97% homology to GLP-1Once Weekly InjectionGLP-1 dimerCARhodes GSK-CRS 18APR2017Lira plus optimizedAlbumin binderTwo GLP-1s on Fc fragment25
Exenatide Oral Formulation ApproachesOramed Lipid Nanoparticle Liver Targetting26
Exenatide Albumin Binding PeptideAmylin Collaboration with Affibody AbIV PK for Analogues in MonkeyOral PK for Analogues in MonkeyLevy OE, Jodka CM, Ren SS, Mamedova L, Sharma A, et al. (2014) Novel ExenatideAnalogs with Peptidic Albumin Binding Domains: Potent Anti-Diabetic Agents withExtended Duration of Action. PLoS ONE 9(2): e87704.doi:10.1371/journal.pone.008770427
Take Home Message for Drug Product DevelopmentIntegration of molecular properties, formulation, and deviceis key to achieving the desired product profileProduct use and self-administration contraints drivedevice configuration, formulation design, molecular properties28
Have Fun and Ask Questions29
The Pharmaceutical Technology Specialistswww.drugdeliveryexperts.com
ExenatideMicrosphere (Bydureon) Life Cycle Bydureon is an exenatide microsphere formulation Vial and syringe, pen, suspension in auto-injector Bydureon (EU 2011 US 2012) Once weekly SC injection 2 mg per week dose Bydureon Pen (US 2014) Once weekly SC injection 2 mg per week dose Bydureon