Transcription
SeminarDeep vein thrombosis and pulmonary embolismMarcello Di Nisio*, Nick van Es*, Harry R BüllerLancet 2016; 388: 3060–73Published OnlineJune 30, 1*Contributed equallyDepartment of Medical, Oraland Biotechnological Sciences,Gabriele D’AnnunzioUniversity, Chieti, Italy(M Di Nisio MD); andDepartment of VascularMedicine, Academic MedicalCentre, Amsterdam,Netherlands (M Di Nisio,N van Es MD, H R Büller MD)Correspondence to:Dr Marcello Di Nisio, Departmentof Medical, Oral andBiotechnological Sciences,Gabriele D’Annunzio University,Chieti, Italymdinisio@unich.itDeep vein thrombosis and pulmonary embolism, collectively referred to as venous thromboembolism, constitute amajor global burden of disease. The diagnostic work-up of suspected deep vein thrombosis or pulmonary embolismincludes the sequential application of a clinical decision rule and D-dimer testing. Imaging and anticoagulation can besafely withheld in patients who are unlikely to have venous thromboembolism and have a normal D-dimer. All otherpatients should undergo ultrasonography in case of suspected deep vein thrombosis and CT in case of suspectedpulmonary embolism. Direct oral anticoagulants are first-line treatment options for venous thromboembolism becausethey are associated with a lower risk of bleeding than vitamin K antagonists and are easier to use. Use of thrombolysisshould be limited to pulmonary embolism associated with haemodynamic instability. Anticoagulant treatment shouldbe continued for at least 3 months to prevent early recurrences. When venous thromboembolism is unprovoked orsecondary to persistent risk factors, extended treatment beyond this period should be considered when the risk ofrecurrence outweighs the risk of major bleeding.IntroductionDeep vein thrombosis and pulmonary embolism aremanifestations of venous thromboembolism. Althoughdeep vein thrombosis develops most often in the legs, thedeep veins of the arms, the splanchnic veins, and thecerebral veins can be affected. In this Seminar we focuson the epidemiology, diagnosis, and treatment of deepvein thrombosis of the legs and pulmonary embolism.Prevention of venous thromboembolism is outside thescope of this Seminar.EpidemiologyVenous thromboembolism is a major global burdenwith about 10 million cases occurring every year, therebyrepresenting the third leading vascular disease afteracute myocardial infarction and stroke.1 Just under halfa million deep vein thromboses and 300 000 pulmonaryembolisms occur every year in six European countrieswith 300 million inhabitants.2 The yearly economicburden of venous thromboembolism in the USA hasbeen estimated to be US 7–10 billion.3 Incidence issteadily increasing because of population ageing, ahigher prevalence of comorbidities associated withvenous thromboembolism, such as obesity, heartfailure, and cancer, and the improved sensitivity andSearch strategy and selection criteriaWe searched MEDLINE, Embase, and the Cochrane Library forpapers published in English from Dec 1, 2009, to March 31,2016, using combinations of the following terms: “deep veinthrombosis”, “pulmonary embolism”, “venousthromboembolism”, “epidemiology”, “diagnosis”,“prognosis”, and “treatment”. We gave preference topublications from the past 5 years, but did consider highlyregarded older publications. We screened the reference lists ofarticles identified by the search strategy and included thosejudged relevant. Pertinent reviews are cited to providereaders with more details and references than we are able toaddress in this Seminar.3060widespread use of imaging tests to detect venousthromboembolism.1,4The annual average incidence increases exponentiallywith age to up to one case per hundred people older than80 years.1,5,6 From age 45 years onwards, the lifetime risk ofdeveloping venous thromboembolism is 8%.1,7 Comparedwith white individuals, incidence is higher in black people8and lower in Asian people,1 a disparity for which cause hasnot yet been elucidated. Risk does not differ by sex,although it seems to be two-times higher in men than inwomen when venous thromboembolisms related topregnancy and oestrogen therapy are not considered.9Venous thromboembolism is associated withsubstantial morbidity and mortality. Although the 30 daymortality rate after pulmonary embolism is decreasing,10,11about 20% of patients with pulmonary embolism still diebefore diagnosis or shortly thereafter, particularly if theembolism is associated with haemodynamic instability.12Long term, venous thromboembolism is a chronicdisease and about 30% of all patients with venousthromboembolism have a recurrence within 10 years.6,13The sequelae of venous thromboembolism are alsoassociated with substantial disability and include thepost-thrombotic syndrome, which develops in 20–50% ofpatients with deep vein thrombosis,14 and chronicthromboembolic pulmonary hypertension, whichcomplicates 0·1–4·0% of pulmonary embolisms.15Although our knowledge of risk factors has increasedover the past decades, a third to a half of venousthromboembolism episodes do not have an identifiableprovoking factor and are therefore classified asunprovoked.16 The remaining episodes are caused(provoked) by transient or persistent factors thatadditively or multiplicatively increase the risk of venousthromboembolism by inducing hypercoagulability,stasis, or vascular wall damage or dysfunction (panel).6,17Strong risk factors for venous thromboembolism includesurgery, immobilisation, and cancer. Risk is especiallyhigh for patients undergoing major orthopaedic surgery;with postoperative rates of around 1% despitepharmacological thromboprophylaxis.18 About 20% of allwww.thelancet.com Vol 388 December 17/24/31, 2016
Seminarvenous thromboembolisms are cancer-related,19 whereassurgery and immobilisation both account for 15% ofcases.5 The most frequent heritable risk factors besidesnon-0 blood group are the factor V Leiden andprothrombin gene mutations, which have a prevalence inthe European population of 3–7% and 1–2%, respectively.20Since heritable risk factors only slightly predict recurrentvenous thromboembolism, thrombophilia testing seemsto have limited or no relevance for the long-termmanagement of venous thromboembolism. Althoughthe list of genetic determinants of venousthromboembolism is constantly updated and evidence isemerging to support testing several single nucleotidepolymorphisms in a single chip, they do not haverelevance yet for clinical practice.21DiagnosisClinical presentationClinical manifestations of deep vein thrombosis of thelegs include swelling or pitting oedema, redness,tenderness, and presence of collateral superficial veins.Signs and symptoms of pulmonary embolism comprisesudden onset of dyspnoea or deterioration of existingdyspnoea, chest pain, syncope or dizziness due tohypotension or shock, haemoptysis, tachycardia, ortachypnoea. Abnormalities on chest radiography,electrocardiography, or blood gas analysis are not specificfor pulmonary embolism, but might be useful in thedifferential diagnosis. About 70% of patients withsymptomatic pulmonary embolism have concomitantdeep vein thrombosis, which is symptomatic in up to aquarter of cases.6,13 Conversely, silent pulmonaryembolism is present in at least a third of patients withsymptomatic deep vein thrombosis.22The great challenge in the diagnostic investigation ofsuspected venous thromboembolism is to accurately andrapidly identify patients in whom prompt treatment isneeded to prevent thrombus extension or embolisation,from patients without disease, in whom unnecessarydiagnostic tests and anticoagulant therapy should beavoided. The diagnosis of venous thromboembolism onthe basis of clinical manifestations alone is unreliablebecause of the poor specificity of signs and symptoms.23Imaging is therefore warranted to confirm or refute thediagnosis. However, among patients with clinicallysuspected deep vein thrombosis or pulmonary embolism,the prevalence of the disease is only about 20%; with abroad variation across countries and clinical settings(range 4–44%).24–26 It is therefore undesirable to imageevery patient with suspected venous thromboembolismbecause of the potential harms of these procedures,including radiation exposure and the risk of contrastinduced nephropathy, as well as associated health-carecosts and use. To guide decisions about who should bereferred for imaging, diagnostic algorithms consisting ofclinical probability assessment and D-dimer testing havebeen established.www.thelancet.com Vol 388 December 17/24/31, 2016Panel: Risk factors for venous thromboembolismClinical and environmental risk factorsHypercoagulability Older age Active cancer Antiphospholipid syndrome Oestrogen therapy Pregnancy or puerperium Personal or family history of venous thromboembolism Obesity Autoimmune and chronic inflammatory diseases (eg,inflammatory bowel disease) Heparin-induced thrombocytopeniaVascular damage Surgery Trauma or fracture Central venous catheter or pacemakerVenous stasis or immobilisation Hospitalisation for acute medical illness Nursing-home residence Long-haul travel for more than 4 h Paresis or paralysisHeritable risk factors Factor V Leiden Prothrombin 20210G A mutation Antithrombin deficiency Protein C deficiency Protein S deficiency Non-0 blood groupClinical probability assessment and D-dimer testingClinical decision rules, which are based on clinicalprobability scores, are used to stratify patients and guidethe selection and interpretation of further diagnostic tests.The Wells’ deep vein thrombosis score consists of tenitems and is the most frequently used score in clinicalpractice for patients with suspected deep vein thrombosis(table 1).32 The best validated scores for suspectedpulmonary embolism are the Wells’ pulmonaryembolism28 and revised Geneva scores,30 which incorporaterisk factors for venous thromboembolism and signs andsymptoms of pulmonary embolism (appendix). Thesescores have been modified over the years to simplify formance.33,34 Although clinical decision rules seem tohave similar performance as empirical clinical evaluation,25they are preferred to standardise clinical assessment andincrease reproducibility among less experiencedphysicians. The Wells’ scores and revised Geneva ruleswere originally intended as three-level rules (low,intermediate, or high clinical probability), but are nowmostly used dichotomously, classifying patients as venousthromboembolism likely or high probability versusSee Online for appendix3061
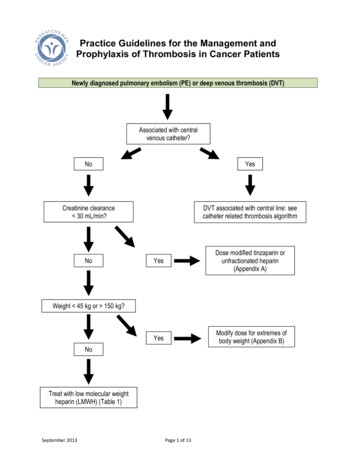
SeminarOriginalpointsSimplifiedpointsActive cancer 1NAParalysis, paresis, or recent plaster cast on lower extremities 1NARecent immobilisation 3 days or major surgery within the past 4 weeks 1NALocalised tenderness of deep venous system 1NAWells’ score for deep vein thrombosis27*Swelling of entire leg 1NACalf swelling 3 cm compared to asymptomatic side 1NAUnilateral pitting oedema 1NACollateral superficial veins 1NAPreviously documented deep vein thrombosis 1NAAlternative diagnosis at least as likely as deep vein thrombosis–2NAWells’ score for pulmonary embolism28,29†‡Alternative diagnosis less likely than pulmonary embolism 3 1Clinical signs and symptoms of deep vein thrombosis 3 1Heart rate 100 beats per min 1·5 1Previous deep vein thrombosis or pulmonary embolism 1·5 1Immobilisation or surgery within the past 4 weeks 1·5 1Active cancer 1 1Haemoptysis 1 1Heart rate 95 beats per min 5 2Heart rate 75–94 beats per min 3 1Pain on lower-limb deep venous palpation and unilateral oedema 4 1Unilateral lower-limb pain 3 1Revised Geneva score for pulmonary embolism30,31§¶Previous deep vein thrombosis or pulmonary embolism 3 1Active cancer 2 1Haemoptysis 2 1Surgery or fracture within the past 4 weeks 2 1Age 65 years 1 1*Classification for original Wells’ score for deep vein thrombosis: deep vein thrombosis unlikely if score 2; deep veinthrombosis likely if score 2. †Classification for original Wells’ score for pulmonary embolism: pulmonary embolismunlikely if score 4; pulmonary embolism likely if score 4. ‡Classification for simplified Wells’ score for pulmonaryembolism: pulmonary embolism unlikely if score 1; pulmonary embolism likely if score 1. §Classification for originalrevised Geneva score for pulmonary embolism: non-high probability of pulmonary embolism if score 10; highprobability of pulmonary embolism if score 10. ¶Classification for simplified revised Geneva score for pulmonaryembolism: non-high probability of pulmonary embolism if score 4; high probability of pulmonary embolism if score 4.Table 1: Clinical decision rules for deep vein thrombosis and pulmonary embolismvenous thromboembolism unlikely or non-highprobability (table 1).35Since clinical decision rules cannot safely exclude thediagnosis of deep vein thrombosis or pulmonaryembolism alone,25 they have to be used in conjunctionwith D-dimer testing. In patients who are thoughtunlikely to have venous thromboembolism based on theclinical decision rule, the diagnosis can be safely excludedbased on a normal D-dimer level (below the definedthreshold value for the test).25,26 With this approach,imaging and treatment can be withheld in approximatelya third of patients with suspected deep vein thrombosisor pulmonary embolism, of whom less than 1% sm in the following 3 months, which isconsidered an acceptable rate.25,263062Quantitative D-dimer assays have a higher sensitivity,but lower specificity, than qualitative tests,36 whichresults in fewer false negative results at the cost of morepatients being referred for imaging.25 Point-of-careD-dimer tests can be done immediately at the emergencydepartment or in the physician’s office and provideresults within 10–15 min. These tests also seem to safelyexclude venous thromboembolism in combination withclinical decision rules,37 which potentially simplifies thediagnostic work-up in the primary care setting andreduces the need for referral to secondary care.38 Tooptimise the trade-off between sensitivity and specificity,the local prevalence of venous thromboembolism shouldbe taken into account when a particular clinical decisionrule and type of D-dimer assay is chosen, since testcharacteristics can vary across different clinicalsettings.24,25 In patients classified as venousthromboembolism likely by the clinical decision rule,the negative predictive value of D-dimer testing isreduced,28,39 and these patients should therefore bereferred for imaging directly (figure 1).The performance of clinical decision rules and D-dimertesting varies across high-risk subgroups. For example, alower specificity for both clinical decision rules andD-dimer assays has consistently been shown in patientswith cancer and in hospitalised patients.26,40–43 As aconsequence, the diagnostic algorithm yields more falsepositive results and a lower proportion of patients inwhom imaging can be withheld. Moreover, in patientswith cancer, ruling out deep vein thrombosis based on avenous thromboembolism unlikely classification andnormal D-dimer test has been found to be neither safenor efficient.26 Therefore, in these subgroups, physiciansmight consider proceeding to imaging directly. Amongpatients with previous venous thromboembolism, avenous thromboembolism unlikely classification incombination with a normal D-dimer safely rules outvenous thromboembolism, but more patients needimaging.26,44 Although a specific clinical decision rule hasbeen proposed for pregnant women with suspected deepvein thrombosis, external validation is needed beforebroader application.45D-dimer levels naturally increase with age and olism is therefore lower in older people. Toincrease the usefulness of D-dimer in these patients, anage-adjusted D-dimer threshold, defined as a patient’sage times 10 μg/L, was derived for patients older than50 years.46 Compared with the conventional, fixedthreshold of 500 μg/L, the age-adjusted threshold has ahigher specificity and similar sensitivity across all agecategories above 50 years,46,47 thereby increasing theabsolute proportion of patients in whom imaging can besafely withheld by 5–6%.46,48 The safety of this ageadjusted D-dimer threshold was recently validated in alarge prospective study of 3346 outpatients with clinicallysuspected pulmonary embolism.33www.thelancet.com Vol 388 December 17/24/31, 2016
SeminarThe performance of the age-adjusted D-dimerthreshold in patients with clinically suspected deep veinthrombosis is under investigation.Another proposed approach for patients with suspectedpulmonary embolism is the application of the PulmonaryEmbolism Rule-out Criteria (PERC) in patients with alow clinical probability according to a three-level clinicaldecision rule.42 If such a low-risk patient meets all PERCcriteria, physicians can refrain from D-dimer testing andconsider the disease excluded.49,50 However, the safety ofthis strategy has not yet been validated in a prospectivemanagement study. Others have proposed a D-dimerthreshold that varies according to the pretest probabilityor even a single D-dimer test to exclude venousthromboembolism, but these strategies also awaitconfirmation.Generally, the use of clinical decision rules and D-dimertesting standardises the diagnostic work-up for venousthromboembolism, reduces the use of invasive tests, andis cost-effective.51 Familiarity with and implementation ofclinical decision rules are important, because inadequateuse can result in inappropriate management and a higherrisk of fatal or non-fatal venous thromboembolism.52,53Imaging for deep vein thrombosisPatients who are likely to have deep vein thrombosisaccording to the Wells’ deep vein thrombosis score, andthose classified as deep vein thrombosis unlikely on thescore but with a D-dimer higher than the conventionalfixed threshold should be referred for diagnostic imaging(figure 1). Compression ultrasonography has replacedcontrast venography as the preferred method for thediagnosis of deep vein thrombosis. Compressionultrasonography is done following two main approaches:whole-leg compression ultrasonography evaluates theentire deep vein system from the groin to the calf,whereas only the popliteal and femoral vein segmentsare imaged with limited (two-point) compressionultrasonography. In patients with an initial normallimited-compression ultrasonography, the examinationshould be repeated after 1 week to ascertain that distal (ie,below-knee) deep vein thrombosis has not propagatedproximally.54 Whole-leg and limited compressionultrasonography are considered equivalent in terms ofsafety since large management studies show bothapproaches to yield false negative results below 1%.55–61The decision to use one approach over the other variesby centre and needs to take into consideration theadvantages and disadvantages of both approaches as wellas the available expertise and facilities. Whole-legcompression ultrasonography is completed in about10–15 min when done by experienced personnel, withgood inter-observer agreement62 and only a fewinconclusive results.59,61 It allows exclusion of bothproximal and distal deep vein thromboses in a singleevaluation and helps with the differential diagnosis ifnone are detected.59,62 The use of whole-leg compressionwww.thelancet.com Vol 388 December 17/24/31, 2016Clinically suspected deep vein thrombosis orpulmonary embolismClinical decision rule*Deep vein thrombosis or pulmonary embolismunlikely†Deep vein thrombosis or pulmonary embolismlikely†D-dimer testing‡NormalAbnormalCUS for deep vein thrombosis or CTPA forpulmonary embolismNegativeDeep vein thrombosis or pulmonary embolismexcludedPositiveDeep vein thrombosis or pulmonary embolismconfirmedFigure 1: Diagnostic algorithm for suspected deep vein thrombosis and pulmonary embolismCTPA computed tomography pulmonary angiography. CUS compression ultrasonography. *Wells’ deep veinthrombosis score for suspected deep vein thrombosis and Wells’ pulmonary embolism score or revised Geneva scorefor suspected pulmonary embolism. †Patients are classified as non-high probability or high probability of deep veinthrombosis by the revised Geneva score, whereas the Wells’ deep vein thrombosis and pulmonary embolism scoresclassify patients as unlikely or likely to have deep vein thrombosis or pulmonary embolism, respectively.‡Fixed ( 500 μg/L) D-dimer testing in patients with suspected deep vein thrombosis and fixed or age-adjusted(age 10 μg/L in patients older than 50 years) D-dimer testing in patients with suspected pulmonary embolism.ultrasonography in all symptomatic patients is associatedwith a 4–15% absolute increase in the diagnosis of deepvein thrombosis due to the detection of isolated clots inthe deep calf veins.63 The prognostic relevance of theseclots remains uncertain and there is controversy abouttheir optimum management.64Limited-compression ultrasonography requires lessexpertise and can be done in 3–5 min in a routine setting.However, a serial examination is required in at least 70% ofpatients, which can be burdensome and is not alwaysfeasible.56,58,60 Moreover, only 1–6% of patients who undergothe second examination are subsequently diagnosed withdeep vein thrombosis.27,60,65 If repeated testing is confinedto the group of patients with both a deep vein thrombosislikely Wells’ score and an abnormal D-dimer, the numberof patients who require serial ultrasonography can bereduced by at least a third.27,66–68 The diagnosis of pelvic orinferior caval deep vein thrombosis can be challengingwith compression ultrasonography and so CT or magneticresonance venography should be considered to exclude thediagnosis in patients with a high clinical suspicion orpregnant women.69About 10% of patients with suspected deep veinthrombosis have a history of deep vein thrombosis.26 Thediagnosis of recurrent deep vein thrombosis by3063
Seminarcompression ultrasonography is hampered by persistingabnormalities of the deep veins in approximately 50% ofpatients 1 year after the initial event, which is reflected bythe poor inter-observer agreement of this test.13,70 Ifcomparison of the residual clot with a previouscompression ultrasonography is not possible or isinadequate, additional tests such as CT venographyshould be considered. Preliminary observations suggesta high accuracy for magnetic resonance direct thrombusimaging with good inter-observer agreement andadequate images in most cases.71recurrent pulmonary embolism in 17% of patients whohad no venous thromboembolism during 3 months offollow-up, whereas the 3 month incidence after a normalCTPA was 3%.43Several other imaging techniques have been evaluatedfor the diagnosis of pulmonary embolism, such as MRI82and single-photon emission CT (SPECT),83 but accuracydata are sparse with limited direct comparisons againstCTPA, and these modalities should therefore at presentbe considered experimental.84Treatment3064Imaging for pulmonary embolismAnticoagulant therapyPatients who are classified as pulmonary embolism likelyby the Wells’ pulmonary embolism score or with a highclinical probability by the revised Geneva score, as well asthose with a D-dimer above the age-adjusted threshold,should be referred for imaging. CT pulmonary angiography(CTPA) has replaced ventilation-perfusion lungscintigraphy and pulmonary angiography as the first-lineimaging test for pulmonary embolism in most centres.CTPA is widely available and modern scanners have a highsensitivity for pulmonary embolism, which allows for itsuse as a stand-alone test.72–74 For example, in two largestudies of pulmonary embolism management, the risk ofvenous thromboembolism at 3 months in patients inwhom anticoagulant therapy was withheld based on anormal CTPA was 0·5% and 1·3%.33,35 Additionally,inadequate scans with CTPA are few (0·6–3·0%) andCTPA can provide an alternative diagnosis whenpulmonary embolism is excluded.75 The use of CTPA asfirst-line imaging for suspected pulmonary embolism canincrease the detection of small, subsegmental pulmonaryembolism, which might have a questionable clinicalrelevance,76 although such isolated peripheral emboli areuncommon.35,77 Ventilation-perfusion lung scanning is assafe as CTPA for diagnosing pulmonary embolism and isassociated with lower radiation exposure,78 but it is oftennot readily available and the test results are non-diagnosticin 30–40% of patients.74 Ventilation-perfusion lungscanning has a role when CTPA is contraindicated becauseof severe renal insufficiency or allergy to contrast medium,and can be considered in pregnant women and youngwomen to reduce radiation exposure to the breast.79 Inhaemodynamically unstable patients with suspectedpulmonary embolism who require a rapid diagnosis ography can be used to disclose signs of rightventricle dysfunction, which could justify emergencyreperfusion.79In patients with suspected recurrent pulmonaryembolism, CTPA is the preferred imaging test.80 Residualpulmonary thrombotic obstruction might complicateinterpretation of imaging tests.81 Nevertheless, in amanagement study of patients with suspected recurrentpulmonary embolism, the combination of an unlikelyclinical decision rule and normal D-dimer safely excludedAnticoagulant therapy is the mainstay for the treatment ofvenous thromboembolism and is classically divided intothree phases: the acute phase of the first 5–10 days aftervenous thromboembolism diagnosis, a maintenancephase of 3–6 months, and an extended phase beyond thisperiod.85 During the acute phase, treatment optionsinclude subcutaneous low-molecular-weight heparin orfondaparinux, intravenous unfractionated heparin, or thedirect oral factor Xa inhibitors rivaroxaban and apixaban(table 2). Unfractionated heparin needs dose adjustmentsbased on activated partial thromboplastin time results,whereas weight-adjusted low-molecular-weight heparinscan be given in fixed doses without monitoring. ionated heparin because of both superior efficacyand safety.86,87 However, unfractionated heparin should beused in patients undergoing thrombolysis because of itsshorter half-life, ease of monitoring, and the possibility toimmediately reverse the anticoagulant effect withprotamine. Unfractionated heparin is also preferred inpeople with severe renal impairment (creatinine clearanceless than 30 mL per min) in whom accumulation oflow-molecular-weight heparin and fondaparinux isexpected given their dependence on renal clearance. Inpatients with suspected or confirmed heparin-inducedthrombocytopenia, heparin should be stoppedimmediately and anticoagulation continued withparenteral anticoagulants such as fondaparinux,argatroban, or lepirudin.88After at least 5 days overlap with vitamin K antagonists,heparins or fondaparinux can be discontinued once theinternational normalised ratio (INR) has repeatedlybeen above 2·0. Vitamin K antagonists have a narrowtherapeutic index due to multiple drug–drug and drug–foodinteractions, which result in substantial interpatient andintrapatient variability. Routine monitoring is thereforerequired to maintain the INR between 2·0 and 3·0.Over the past decade, direct oral anticoagulants,comprising the thrombin inhibitor dabigatran etexilateand the factor Xa inhibitors rivaroxaban, apixaban, andedoxaban, have been introduced for the treatmentof venous thromboembolism. These agents overcomemany disadvantages of vitamin K antagonists. Direct oralanticoagulants have little interaction with otherwww.thelancet.com Vol 388 December 17/24/31, 2016
SeminarUnfractionated heparinRoute ofadministrationRenal clearanceHalf-lifeInitial treatment dosingMaintenancetreatment dosingIntravenous 30% 1·5 hMaintain aPTT 1·5-times upper limit ··of normalExtended treatmentdosing··Low-molecular-weight heparinSubcutaneous 80%3–4 hWeight-based dosingWeight-based dosing*··FondaparinuxSubcutaneous100%17–21 hWeight-based dosingWeight-based dosing··Vitamin K antagonistsOralNegligibleAcenocoumarol 8–11 h;warfarin 36 h;phenprocoumon 160 hTarget at INR at 2·0–3·0 and giveparallel heparin treatment for atleast 5 daysMaintain INR at 2·0–3·0Maintain INR at 2·0–3·0DabigatranOral 80%†14–17 hRequires at least 5 days heparinlead-in150 mg twice a day150 mg twice a dayRivaroxabanOral 33%‡7–11 h15 mg twice a day for 3 weeks20 mg once a day20 mg once a dayApixabanOral 25%‡8–12 h10 mg twice a day for 1 week5 mg twice a day2·5 mg twice a dayEdoxabanOral 35%‡6–11 hRequires at least 5 days heparinlead-in60 mg once a day§60 mg once a day§AspirinOral 10%15 min····80–100 mg once a dayaPTT activated partial thromboplastin time. INR international normalised ratio. *Treatment with low-molecular-weight heparin is recommended for patients with active cancer and pregnant women. †Dabigatran iscontraindicated in patients with a creatinine clearance below 30 mL per min. ‡Apixaban, edoxaban, and rivaroxaban are contraindicated in patients with a creatinine clearance below 15 mL per min.§The recommended edoxaban dose is 30 mg once a day for patients with a creatinine clearance of 30–50 mL per min, a bodyweight less than or equal to 60 kg, or for those on certain strong P-glycoprotein inhibitors.Table 2: Anticoagulant therapies for deep vein thrombosis and pulmonary embolismmedications and food and can be given in fixed doseswithout routine monitoring, hence greatly simplifyingtreatment (table 2). The concurrent use of strongP-glycoprotein inhibitors or potent cytochrome P450 3A4inhibitors or inducers (eg, certain protease inhibitors,antimycotics, and antiepileptic drugs) should be avoidedsince they can influence the exposure to direct oralanticoagulants.89 Direct oral anticoagulants have a rapidonset of action with peak levels reached within 2–4 h anda half-life of about 12 h, which is much shorter than thatof vitamin K antagonists. Whereas vitamin K antagonistsare
Seminar 3060 www.thelancet.com Vol 388 December 17/24/31, 2016 . Long term, venous thromboembolism is a chronic disease and about 30% of all patients with venous . induced nephropathy, as well as associated health-care costs and use. To guide decisions about who should be