Transcription
Management of Anaemia in HD patients –what has PIVOTAL taught us?Iain C. Macdougallon behalf of the PIVOTAL Study GroupRenal Unit, King’s College Hospital, London, UKThis investigator-led clinical trial is supported through an unrestrictedgrant fromUK Kidney Research Consortium :Renal Anaemia CSG
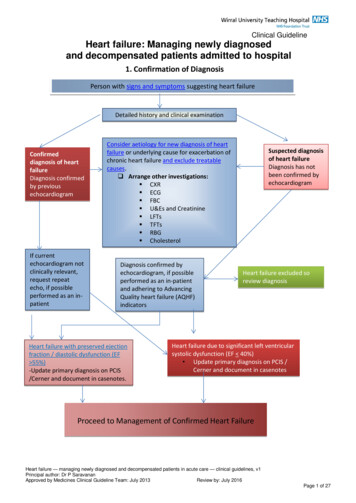
Management of Anaemia in HDESA therapyIV iron
US Normal Hematocrit TrialCHOIRCREATETREAT
19982006HarmNo benefitNormal HematocritCHOIRCREATE2SinghA, et al. NEJM2006;355:2085-98AT 42009Stopped earlyBesarab A, et al. NEJM1998;339:584–590TREATE3CRECHOIR2NHCT 1Outcome trials of ESA therapy3DrüekeT, et al. NEJM2006;355:2071–84No benefit/harmTREAT4PfefferM, et al. NEJM2009;361:2019–32
EPO has non-erythropoietic actionsHigh EPO levels VSMC [Ca2 ]i RAS activation ET-1 ThromboxaneVSMC proliferationEC proliferationAngiogenesis Platelet production Platelet activity E selectin Prostacyclin P selectin ADMA vWF PAI-1 NOBlood access stenosisProliferative retinopathyHypertensionVascular remodelingTumor growthThrombosisVaziri ND & Zhou X. Nephrol Dial Transplant 2009; 24: 1082–1088.
IV iron improves haemoglobinHb (g/dL)14ESA IV ironESA oral ironESA no iron12IV iron****10**********86**Oral iron0481216Time (weeks)Macdougall et al, Kidney Int (1996)Fishbane et al, Am J Kidney Dis (1995)
IV iron reduces EPO dosesOral ironIV ironFishbane et al, Am J Kidney Dis (1995)
-20%DeVita 2003b-12.4%-34.4%-46.5%n 39n 112Kapoian2008n 36Schiesser 2006n 36-17.4%-23.9%-25.0%-30%-40%n 149Chang 2002n 228Richardson 2001-18.5%n 25Descombes 2000n 50Sepandj 1996n 37Macdougall1996-10%Fishbane 1995% change in ESA dose0%n 52DeVita2003aIV iron reduces ESA doses-32.5%-47.1%-49.4%-50%-60%Fishbane S et al. Am J Kidney Dis 1995;26:41–46; Macdougall I et al. Kidney Int 1996;50: 1694–1699; Sepandj F et al. Nephrol Dial Transplant 1996;11:319–322; Descombes E & Fellay G.Nephron 2000;84:196–197; Richardson D et al. Am J Kidney Dis 2001;38:109–117; Chang CH et al. Clin Nephrol 2002;57:136–141; De Vita MV et al. Clin Nephrol 2003;60:335–340;Schiesser D et al. Nephrol Dial Transplant 2006;21:2841–2845; Kapoian T et al. JASN 2008;19:372–379
Haemoglobin, ESA, and IV iron use in US dialysispatients (1992 2005)1. Ibrahim HN et al. Am J Kidney Dis 2008;52:1115–1121;2. Ibrahim HN et al. Nephrol Dial Transplant 2009;24:3138–3143
Iron and oxidative stressFe 2 Fenton reactionFe 3 OH- radicalROSmacromolecules,e.g. membrane lipidslipid-derived free radicalsatherosclerosis
Plasma MDA concentration (µmol/L)Iron increases oxidative stress1.2*1.00.80.60.40.20CTLCTL FeCRFCRF FePlasma malondialdehyde (MDA) levels in control rats (CTL), Fe-injected control rats (CTL Fe), chronicrenal failure rats (CRF), and Fe-injected CRF rats (CRF Fe). (N 6 in each group) P 0.05 vs. CTLgroup.Lim C et al. Kidney Int 2004;65:1802–9.
Associations between IV iron dose and mortality
Association of IV Iron Dose with MortalityCV-Mortality22UnadjustedCase-mixCase-mix & MICS1.5CV-Mortality Hazard RatioAll-Cause Mortality Hazard RatioAll-Cause Mortality10.80.60.401-199200-399 400IV Iron dose (mg/month)MICS malnutrition-inflammation-cachexia syndrome.Kalantar-Zadeh K, et al. J Am Soc Nephrol. 2005;16(10):3070-3080.UnadjustedCase-mixCase-mix & MICS1.510.80.60.401-199200-399 400IV Iron dose (mg/month)
Ferritin and IV Iron Use in DOPPSMean Serum Ferritin (ng/mL)Mean IV Iron Dose 0th25th10th20010002006 20072010 2011 2012Study YearANZN Facilities: 8BE16CA13FR15GE20IT10JP14SP15SW16UK14DOPPS 4 Cross Section, Vintage 90 days,Facilities with 10 PatientsBailie GR, et al. Nephrol Dial Transplant. 2013;28(10):2570-2579.US96
KDIGO 2014 Controversies ConferenceSan Francisco, 27–30 March, 2014IRON MANAGEMENT IN CKD
Iron Management in CKD ConferenceSteering CommitteeGlenn Chertow, USA – Conference Co-ChairIain Macdougall, UK – Conference Co-ChairIron Overload Co-ChairsKai-Uwe Eckardt, Germany & Dorine Swinkels, NetherlandsInflammation & Oxidative Stress Co-ChairsPeter Stenvinkel, Sweden & Christoph Wanner, GermanyIron & Infection Co-ChairsGregorio Obrador, Mexico & Günter Weiss, AustriaHypersensitivity Reactions to IV Iron Co-ChairsAndreas Bircher, Switzerland & Carol Pollock, Australia
Macdougall et al. Kidney Int 2016; 89 : 28-39.
PIVOTALProactive IV irOn Therapy in haemodiALysis
PIVOTAL Steering CommitteeIain Macdougall, Claire White, Stefan Anker, Sunil Bhandari, Kenneth Farrington,Philip Kalra, John McMurray, Heather Murray, Charles Tomson, David Wheeler,Christopher Winearls, Ian FordEndpoint Adjudication Committee (chair: John McMurray)Independent Data Monitoring Board (chair: Alan Jardine)Kidney Research UK (Elaine Davies; Michael Nation)Vifor Fresenius Medical Care Renal Pharma Ltd (Sandra Wächter)Editorial support from Adam Perahia NorthStar Strategic Consulting
Network of Sites
N Engl J Med 2019 Jan 31;380: 447-458.
Hypothesis: Proactive, high-dose IV iron would be non-inferior to reactive,low-dose IV iron for the outcome of all cause mortality and cardiovascularevents in haemodialysis patients.Prospective Randomised, Open label, Blinded Endpoint (PROBE) designEudra CT: 2013-002276-25
Trial DesignProactive, high-dose IV iron arm (n 1093)n 2589New to HD(0-12 months)On ESAFerritin 400 µg/LTSAT 30%IV iron 400 mg/month (withhold if ferritin 700 μg/L; TSAT 40%)Rn 2141Reactive, low-dose IV iron arm (n 1048)IV iron only administered if ferritin 200 μg/L or TSAT 20%Screening: 4 weeksFollow-up period with monthly visitsMedian (maximum) follow-up: 2.1 (4.4) years 631 primaryendpoint events(i.e., all-causemortality, MI,stroke, or HFhospitalization)
Statistical analysisEvent rate: Assumed 3 year event rate of 40% in placebo group and 10%loss to follow-up (including transplantation).Sample size: Proposed sample size of 2080.Power: 631 primary outcome events required to exclude non-inferiority of ahazard ratio of 1.25 with 80% power.Superiority testing if non-inferiority confirmed.Eudra CT: 2013-002276-25
OutcomesPrimary Composite of nonfatal MI, nonfatal stroke, hospitalization for HF, or all-cause death, analyzed as time-to-first eventSecondary (efficacy)MI, stroke, hospitalization for HF, and deaths(first recurrent events)All-cause deathFirst composite CV event (MI, stroke, and hospitalization for HF)Fatal or nonfatal MIFatal or nonfatal strokeHospitalization for HFESA dose requirementsTransfusion requirementsQuality-of-life measuresCumulative dose of ironHemoglobin concentrationSerum ferritin concentration Secondary (safety)Vascular access thrombosisAll-cause hospitalizationHospitalization for infectionInfection episodesTertiary Platelet count Serum albumin concentration TSAT
Patient DispositionAssessed for eligibility (n 2589)Excluded (n 448)Randomized(n 2141)Randomized to reactive, low-dose IV ironIntention-to-treat (ITT) population (n 1048)Per-protocol (PP) population (n 1038)Excluded (n 10) Current malignancy (n 7) Elevated serum ferritin (n 3)Incomplete follow-up (n 631) Death (n 269) Transplant (n 187) Moved to home dialysis (n 36) Moved to peritoneal dialysis (n 17) Lost to follow-up (n 78) Withdrew from study (n 44)Included in ITT analysis (n 1048)Included in PP analysis (n 1038)Included in safety analyses (n 1048)Randomized to proactive, high-dose IV ironIntention-to-treat (ITT) population (n 1093)Per-protocol (PP) population (n 1080)Excluded (n 13) Current malignancy (n 12) Elevated serum ferritin (n 1)Incomplete follow-up (n 592) Death (n 246) Transplant (n 184) Moved to home dialysis (n 45) Moved to peritoneal dialysis(n 13) Lost to follow-up (n 62) Withdrew from study (n 42)Included in ITT analysis (n 1093)Included in PP analysis (n 1080)Included in safety analyses (n 1093)
Baseline CharacteristicsCharacteristicMean (SD) age, yearMale sexMedian (LQ, UQ) dialysis vintage, monthVascular accessDialysis catheterArteriovenous fistula/graftCardiovascular diseaseAtrial fibrillationHeart failureHypertensionHyperlipidemiaPeripheral vascular diseasePrior myocardial infarctionPrior strokeDiabetesCurrent smokerProactive, High-Dose IV Iron(N 1093)62.7 (14.9)65.0%4.9 (2.8, 8.4)Reactive, Low-Dose IV Iron(N 1048)62.9 (15.1)65.6%4.8 (2.8, .9%P 0.03
Baseline Characteristics (cont’d)Proactive, High-Dose IV Iron(N 1093)Reactive, Low-Dose IV Iron(N 1048)Body-mass index — kg/m2, mean (SD)28.5 (7.1)29.0 (6.7)Systolic BP — mm Hg, mean (SD)a145 (24)145 (24)Diastolic BP — mm Hg, mean (SD)a74 (14)74 (15)10.6 (1.4)10.5 (1.4)214 (132, 305)217 (137, 301)20 (16, 24)20 (16, 24)6.0 (3.3, 13.9)7.0 (4.0, 15.0)8000 (5000, 10,000)8000 (5000, 12,000)ACE inhibitor/ARB at baseline25.3%30.3%P 0.009Phosphate binders at baseline36.0%40.9%P 0.02CharacteristicHemoglobin — g/dL, mean (SD)Serum ferritin — μg/L, median (LQ, UQ)TSAT — %, median (LQ, UQ)CRP — mg/L, median (LQ, UQ)ESA dose — IU/wk, median (LQ, UQ)bP 0.04
Baseline ESAEpoetin alfa25,1%Epoetin beta17,3%Epoetin theta0,3%Darbepoetin alfa53,6%Methoxy polyethylene glycol-epoetin beta3,7%
Cumulative Iron Dose11,000Mean Cumulative IV Iron (mg)10,000Proactive, high-dose iron9000P 0.00180007000Median cumulative dosesat 1 year: 3.8 g vs 1.8 g60005000Reactive, low-dose iron40003000Median monthly doses:264 mg vs 145 mg200010000036912151821242730Time from Randomization (months)3336394245
Serum Ferritin Concentration750700Mean Ferritin (μg/L)650600Proactive, high-dose iron550500450P 0.001(Treatment effect)400350300Reactive, low-dose iron250200150100036912151821242730Time from Randomization (months)3336394245
Transferrin Saturation30Mean TSAT (%)28Proactive, high-dose iron26P 0.001(Treatment effect)2422Reactive, low-dose iron2018036912151821242730Time from Randomization (months)3336394245
Mean Cumulative ESA Dose (1000 IU)Cumulative ESA Dose21001800P 0.0115001200Reactive, low-dose iron900Median monthly dosesreduced by 19.4%600Proactive, high-dose iron3000036912151821242730Time from Randomization (months)3336394245
40Hazard ratio, 0.85 (95% CI, 0.73–1.00)Noninferiority P 0.001Superiority P 0.04Reactive, low-dose iron20Proactive, high-dose iron0Patients with Event (%)60Death, MI, Stroke, or HF Hospitalization(Primary Endpoint)00,511,5Time (years)22,533,5
Subgroup Analysis: Primary EndpointSubgroupProactive, HighDose IronReactive, LowDose IronP Value forInteractionHazard Ratio (95% CI)no. of events/total no. (%)All participants320/1093 (29.3)0.85 (0.73–1.00)338/1048 (32.3)Duration of dialysis treatment at enrollment0.79 5 months139/499 (27.9)146/487 (30.0)0.88 (0.69–1.11) 5 months181/594 (30.5)192/561 (34.2)0.84 (0.68–1.03)Dialysis access0.22Dialysis catheter137/452 (30.3)159/432 (36.8)0.77 (0.61–0.97)AV fistula183/641 (28.6)179/616 (29.1)0.93 (0.76–1.14)Baseline diabetes0.62Yes182/490 (37.1)193/453 (42.6)0.83 (0.67–1.01)No138/603 (22.9)145/595 (24.4)0.90 (0.71–1.13)0.60.70.80.91.0Proactive, High-Dose Better1.1 1.2Reactive, Low-Dose Better
Primary Endpoints per 100 PatientsPrimary Endpoint Componentsaas Recurrent Events60Rate ratio, 0.77 (95% CI, 0.66–0.92)P 0.002740Reactive, low-dose ironProactive, high-dose iron200012Time (years)aDeathfrom any cause, MI, stroke, and hospitalization for HF.Recurrent events plotted in the form of mean frequency functions using the method of Ghosh and Lin (Biometrics. 2000;56:554-562.).3
Patients with Event (%)Death from Any CauseHazard ratio, 0.84 (95% CI, 0.71–1.00)P 0.05440Reactive, low-dose ironProactive, high-dose iron20000,511,52Time (years)2,533,5
Subgroup Analysis: All-cause DeathSubgroupProactive, HighDose IronReactive, LowDose IronP Value forInteractionHazard Ratio (95% CI)no. of events/total no. (%)All participants246/1093 (22.5)0.84 (0.71–1.00)269/1048 (25.6)Duration of dialysis treatment at enrollment0.99 5 months103/499 (20.6)116/487 (23.8)0.84 (0.65–1.10) 5 months143/594 (24.1)153/561 (27.3)0.84 (0.67–1.06)Dialysis access0.18Dialysis catheter102/452 (22.6)127/432 (29.4)0.74 (0.57–0.96)AV fistula144/641 (22.5)142/616 (23.1)0.94 (0.74–1.18)Baseline diabetes0.93Yes138/490 (28.2)149/453 (32.9)0.83 (0.66–1.05)No108/603 (17.9)120/595 (20.2)0.86 (0.66–1.11)0.60.70.80.91.0Proactive, High-Dose Better1.1 1.2Reactive, Low-Dose Better
Cardiovascular EventsProactive, HighDose IV Iron(N 1093)n (%)Reactive, LowDose IV Iron(N 1048)n (%)149 (13.6)168 (16.0)0.80(0.64–1.00)0.049Fatal or nonfatal MI78 (7.1)102 (9.7)0.69(0.52–0.93)0.015Fatal or nonfatal stroke34 (3.1)35 (3.3)0.90(0.56–1.44)0.663Hospitalization for HF51 (4.7)70 (6.7)0.66(0.46–0.94)0.023OutcomeFatal or nonfatal MI, fatal or nonfatalstroke, or hospitalization for HFHazard Ratio(95% CI)P Value
Hazard ratio, 0.69 (95% CI, 0.52–0.93)P 0.015Numbers at 22
Hazard ratio, 0.90 (95% CI, 0.56–1.44)P 0.663Numbers at 22
Hazard ratio, 0.66 (95% CI, 0.46–0.94)P 0.023Numbers at 22
Hemoglobin Concentration12.0Mean Hemoglobin (g/dL)11.811.6Proactive, high-dose iron11.411.211.010.810.6Reactive, low-dose iron10.4P 0.001 through month 12(Treatment effect)10.210.0036912151821242730Time from Randomization (months)3336394245
Blood TransfusionsPatients with Event (%)40Hazard ratio, 0.79 (95% CI, 0.65–0.95)P 0.014Reactive, low-dose iron20Proactive, high-dose iron000,511,52Time (years)2,533,5
SafetyEndpointProactive,High-DoseIV Iron(N 1093)n (%)Reactive,Low-DoseIV Iron(N 1048)n (%)Vascular access thrombosis262 (24.0)218 (20.8)1.15 (0.96–1.38)0.12All-cause hospitalization651 (59.6)616 (58.8)1.01 (0.90–1.12)0.90Hospitalization for infection323 (29.6)307 (29.3)0.99 (0.82–1.16)0.92Infection episodes508 (46.5)477 (45.5)0.98 (0.87–1.11)0.80Hazard Ratio (95% CI)0.80.91.0Proactive, High-Dose Better1.11.21.31.4Reactive, Low-Dose BetterP Value
ConclusionsIn patients undergoing maintenance HD, a proactive, high-doseregimen of IV iron (relative to a reactive, low-dose regimen): Significantly reduced the risk of the primary outcome of deathor nonfatal CV events Reduced the risk of MI and hospitalization for HF Was associated with a significant benefit in a recurrentevent analysis Reduced ESA dose (19.4%) and transfusion rate (21%) Did not cause an increased risk of infection or hospitalization
Management of Anaemia in HD patients - what has PIVOTAL taught us? Iain C. Macdougall on behalf of the PIVOTAL Study Group. Management of Anaemia in HD ESA therapy IV iron. CREATE CHOIR US Normal Hematocrit Trial . New to HD (0-12 months) On ESA Ferritin 400 µg/L TSAT 30% 631 primary endpoint events (i.e., all-cause mortality, MI .