Transcription
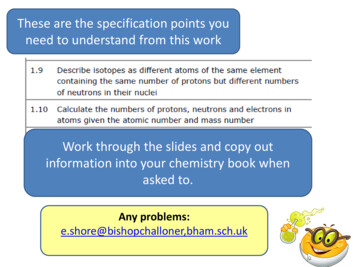
These are the specification points youneed to understand from this workWork through the slides and copy outinformation into your chemistry book whenasked to.Any problems:e.shore@bishopchalloner,bham.sch.uk
Starter To begin with, let us seehow much you rememberfrom last week’s lesson.
You will need to use this from last lesson:
Atomic structure revision4 of 19 Boardworks Ltd 2011
Answers5 of 19 Boardworks Ltd 2011
IsotopesObjectives: To know how to work out the numbers ofprotons, neutrons and electrons in an atom. To understand what isotopes are. To be able to work out the number of p,n andelectrons in different isotopes. To be able to answer question.
What is an isotope?Copy into your bookWhat do atoms of the same element always have the samenumber of?protonsAtoms of an element can come in slightly different forms.Atoms that differ in this way are called isotopes.For example, two isotopes of carbon:mass numberis differentatomic numberis the same7 of 19 Boardworks Ltd 2011
Copy the purple writing into your bookThese two isotopes are called:carbon-12 and carbon-13. The number written is themass number.This is not a minus sign, it is a hyphenFor example, two isotopes of carbon:mass numberis differentatomic numberis the same8 of 19 Boardworks Ltd 2011
What is an isotope?What is different about the two isotopes, the number ofprotons, neutrons or electrons ?For example, two isotopes of carbon:mass numberis differentatomic numberis the same9 of 19 Boardworks Ltd 2011
Copy the purple writing into your bookProtons: the atomic number is the same for both, this meansthe number of protons is the same.Electrons:remember that in an atom, the number of electrons isthe same as the number of protons, so the number of electronsis the same for both.Neutrons: mass number protons neutrons.The mass number is different for the two isotopes and as thenumber of protons stays the same, it must be the number ofneutrons that is different.For example, two isotopes of carbon:mass numberis differentatomic numberis the same10 of 19 Boardworks Ltd 2011
Whatis anisotope?Copy thepurplewriting into your book.You need to learn this definition.DefinitionIsotopes are atoms of the same element with the samenumber of protons and electrons but a differentnumber of neutrons.For example, two isotopes of carbon:mass numberis differentatomic numberis the same11 of 19 Boardworks Ltd 2011
If you have internet access, watch thefollowing Bitesize video: https://www.bbc.co.uk/bitesize/clips/z89n34j12 of 19 Boardworks Ltd 2011
Copy into your bookThe isotopes of an element are virtually identical in theirchemical reactions.This is because theyall have the samenumber of protonsand the samenumber of electrons.The uncharged neutrons make little difference to chemicalproperties, but do affect physical properties such as meltingpoint and density.Natural samples of elements are often a mixture of isotopes.13 of 19 Boardworks Ltd 2011
IsotopesObjectives: To know how to work out the numbers ofprotons, neutrons and electrons in an atom. To understand what isotopes are. To be able to work out the number of p,n andelectrons in different isotopes. To be able to answer question.
How many protons, neutrons and electrons arepresent in different isotopes?Most naturally-occurring carbon exists as carbon-12, about1% is carbon-13 and a much smaller amount is carbon-14.6 protons6 neutrons6 electrons15 of 196 protons7 neutrons6 electrons6 protons8 neutrons6 electrons Boardworks Ltd 2011
Can you work out how many protons, neutrons and electronsare present in the below hydrogen isotopes?Hydrogen-1 makes up the vast majority of the naturallyoccurring element, but two other isotopes exist.hydrogen1 proton0 neutrons1 electron16 of 19deuterium1 proton1 neutron1 electrontritium1 proton2 neutrons1 electron Boardworks Ltd 2011
What are the isotopesof hydrogen?AnswersHydrogen-1 makes up the vast majority of the naturallyoccurring element, but two other isotopes exist.hydrogen1 proton0 neutrons1 electron17 of 19deuterium1 proton1 neutron1 electrontritium1 proton2 neutrons1 electron Boardworks Ltd 2011
Canyouareworkoutisotopeshow manyofprotons,neutrons and electronsWhatthechlorine?are present in the below chlorine isotopes?About 75% of naturally-occurring chlorine is chlorine-35, and25% is chlorine-37.18 of 1917 protons17 protons18 neutrons20 neutrons17 electrons17 electrons Boardworks Ltd 2011
What are the isotopesof chlorine?AnswersAbout 75% of naturally-occurring chlorine is chlorine-35, and25% is chlorine-37.19 of 1917 protons17 protons18 neutrons20 neutrons17 electrons17 electrons Boardworks Ltd 2011
Canyouareworkoutisotopeshow manyofprotons,neutrons and electronsWhattheoxygen?are present in the below oxygen isotopes?Almost all naturally-occurring oxygen is oxygen-16, butabout 0.2% is oxygen-18.What are the particle numbers in each isotope below?oxygen-168 protons8 neutrons8 electronsoxygen-188 protons10 neutrons8 electrons20 of 19 Boardworks Ltd 2011
What are the isotopesof oxygen?AnswersAlmost all naturally-occurring oxygen is oxygen-16, butabout 0.2% is oxygen-18.What are the particle numbers in each isotope below?oxygen-168 protons8 neutrons8 electronsoxygen-188 protons10 neutrons8 electrons21 of 19 Boardworks Ltd 2011
IsotopesObjectives: To know how to work out the numbers ofprotons, neutrons and electrons in an atom. To understand what isotopes are. To be able to work out the number of p,n andelectrons in different isotopes. To be able to answer questions.
You will need your periodic table for thenext questions.23 of 19 Boardworks Ltd 2011
Copy out and complete the below table
Answers
Copy out and complete the below table
Answers
Knowledge check Can you answer the following questions?28 of 19 Boardworks Ltd 2011
1.What are isotopes?2. Which number is different for isotopes, atomic number ormass number?3. Why do isotopes of an element have the same chemicalproperty?4. State a particular physical property that might bedifferent for two isotopes of an element?
What are isotopes?Atoms of the same element with the same number ofprotons and electrons but a different number of neutrons.2. Which number is different for isotopes, atomic number ormass number?mass number3. Why do isotopes of an element have the same chemicalproperty?Because it is the electrons and protons of an element thatdetermine the chemical properties of an element and theseare the same in isotopes of an element.4. State a particular physical property that might bedifferent for two isotopes of an element?melting point or boiling point1.
Well done, that is the end ofweek 6 chemistry.
Isotopes Objectives: To know how to work out the numbers of protons, neutrons and electrons in an atom. To understand what iso