Transcription
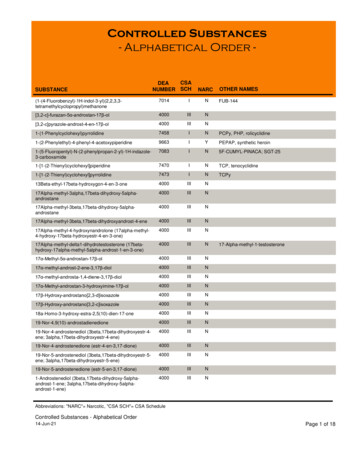
CHAPTER 18CONTROLLED SUBSTANCES18.1
HospitalCONTROLLED SUBSTANCESDEA DEA assigned to facility (vs. the pharmacy) Registrant is entity vs. individual Renewal every 3 years, can renew & manage registration online Director of Pharmacy responsible for oversight Large number of individuals handle controlled substances (CS) Sound P&P to ensure accountability, minimize opportunity for diversionOrdering Schedule II-V DEA form 222 required for ordering CIIs Can request order form books on DEA website; will receive the maximum number oforder form books allowed for your business activity (7 forms per book, typically max 6books per request) Registrant may authorize power of attorney (POA) for individuals responsible for signingschedule II orders (sample form page 18.9). Must be signed by the person who signedmost recent application for registration or renewal plus the individual being authorized. All executed 222 order forms must be dated on or after POA authorization date (shouldnot have 222 orders signed by individuals that don’t have an active POA on file). Maintain POA forms as part of CS records Keep POA records up to dateo If renewal application signed by a different person (new DOP), must update POAfileso Revoke POA when employee leaves or responsibilities change; maintain record ofrevoked POA in files CSOS – electronic schedule II ordering (can also use for II-V ordering) Registrant and POAs can order via CSOS once registered and digital certificateactivated (registrant designates a CSOS coordinator) CSOS certificates expire when registration expires Individual enrollment; must have active POA on file prior to enrolling in CSOS Individual must enroll under each DEA license if ordering for multiple sites CSOS certificate valid for all vendors; separate set up for each No line item limit for ordering, faster than paper 222, accurate Can separate tasks of ordering and signing (added accountability) Schedule III-V - order from wholesaler or vendors ADC system interfacing tracks pars at the cabinet level and communicates to CS vault forinventory management/reorderingReceiving Schedule II-V CS totes delivered directly to vault; separate from non-controlled inventory18.2
Ensure not delivered to loading docks or other general areas Best practice – validate inventory received against invoices before signing for driver and taking custody of shipment (in transit loss is responsibility of supplier; after take custody,responsibility of pharmacy and requires reconciliation with supplier and/or DEA 106 forloss)Confirm receipt of each line item on invoice for all II-VDate/initial each line on 222 formDelegate ordering and receiving to different individualsA third individual should audit receiving records to validate what was ordered to what wasreceived and then stocked into inventory. Compare DEA 222 form (or printed CSOS) to wholesaler invoice Compare wholesaler invoice to vault receipt recordsStorage & Security Locked in a secure area as defined by hospital policy MM 03.01.01 “in a secure area to prevent diversion” Many organizations use automated dispensing cabinets (ADC) to secure controlledsubstances Require individual login Cabinet access restricted to authorized users Can restrict based on role (respiratory therapists can access cabinet and specific bins butcannot access CS or other drugs they don’t administer) Maintains perpetual inventory and transaction record, provides discrepancy notificationsat the cabinet and system level, maintains audit trail Accessing CS pocket requires a “blind count” by users (unlike non-controlled substances,the system doesn’t tell the user what the count should be in the pocket) User notified if count entered is wrong, prompted to recount or create discrepancy May choose to classify other drugs with potential for abuse or diversion as a “control”for tightened security, blind counts, discrepancy tracking and audit trails (propofol,sildenafil) CS only stored in lock lidded bins vs. open matrix, limited access, auditable Pharmacy vault restricted to those with CS responsibilities Users must protect passwords; Bio-ID preferred over password if possible Ensure terminated employees’ access to ADCs is revoked promptly Always log out of machine; auto log-off after set time period with no activity Hospitals without ADCs utilize med carts or locked cabinets on the unit, difficult to maintainsecurity of keys when large number of nurses Emergency kits and code cart trays – ideally don’t contain controlled substances, too easy toaccess or transport out of unit/facility unnoticed Other considerations Securing IV controlled substances in patient rooms, consider lock boxes18.3
PCA and epidural pump key accountability and security Secure delivery of CS to patient care areasWaste With witness as defined by hospital policyDocument all CS wasteWitness login/password required at the ADCManual (paper log, anesthesia records) requires signature of witnessReinforce witness needs to actually witness/visualize wasteReconcile waste transactions (administered wasted dispensed), follow up ondiscrepancies Consider handling of fentanyl patch wasteDisposal/Destruction Reverse Distributor – remove expired CS from site Schedule II transferred via DEA 222 III-V invoiced, maintain records DEA form 41 for destruction CS for immediate administration in an institutional setting remains under the control ofthe institution even if the substance is not fully exhausted (such as partial vial,considered pharmaceutical waste vs. requiring disposal/destruction)64B16-28.303 Destruction of Controlled Substances All Permittees (Excluding Institutional Class I NursingHomes).(1) Controlled substances that cannot be retained as usable shall be securely stored in thepharmacy/prescription department of the permittee pharmacy until destroyed.(2) Permittees are required to complete a United States Drug Enforcement Administration (D.E.A.) Form DEA41 “Registrants Inventory of Drugs Surrendered” (effective 8/31/2014), herein incorporated by reference,available at http://www.flrules.org/Gateway/reference.asp?No Ref-03998 orhttp://www.deadiversion.usdoj.gov/21cfr reports/surrend/. This form, at the time of destruction, shall bewitnessed and signed by the prescription department manager or the consultant pharmacist of record andD.E.A. agent, or a Department inspector. This method of destruction requires that a copy of the completedand witnessed Form DEA 41 be mailed to the D.E.A. office in his/her area within one (1) business day after thedestruction.18.4
(3) Another method of destruction shall be conducted by at least two persons: One will be the prescriptiondepartment manager or the consultant pharmacist of record. The other will be one of the following: medicaldirector or his/her physician designee, director of nursing or his/her licensed nurse designee, or a sworn lawenforcement officer. These persons shall serve as the witnesses for the Form DEA-41 and the destruction. Thismethod of destruction requires that a copy of the completed and witnessed Form DEA-41 be mailed to theD.E.A. office in the permittee’s area within one (1) business day after destruction.(4) In lieu of destruction on the premises as outlined in subsections (2) and (3) above, controlled substancesmay also be shipped to reverse distributors for destruction in conformity with federal guidelines.(5) For patient specific controlled substance prescriptions in a Modified Institutional Class II B pharmacy, thedestruction method in subsection 64B16-28.301(2), F.A.C., must be followed.Rulemaking Authority 465.005, 465.022 FS. Law Implemented 465.022, 465.018 FS. History–New 4-21-87,Formerly 21S-19.003, Amended 7-31-91, Formerly 21S-28.303, 61F10-28.303, Amended 1-30-96, Formerly 59X28.303, Amended 2-5-07, 10-27-09, 2-1-12, 4-20-14. Also refer to pages 18.12-15 FAQ: DEA Rule on Disposal of Controlled SubstancesAccountability Must be able to account for CS through entire system (closed system); maintain audit trail Outside of Pharmacy - Discrepancy monitoring by pharmacy and nursing Policies establishing expectations for surveillance (who, how often, resolution process),see sample table page 18.10 Discrepancies between expected and actual quantity on hand should be resolved in atimely manner with proper documentation of reason by person creating the discrepancyand a witness Unresolved discrepancies monitored by pharmacy and nurse manager or designee(charge nurse) Pharmacy should review discrepancy resolutions for appropriateness Establish an escalation pathway to nursing leadership for discrepancies not resolvedwithin certain time period or with inappropriate reason Empty return bins regularly, monitor for inventory discrepancies (establish who isresponsible for emptying, monitoring) Nursing should have process to ensure what dispensed is documented as administered,wasted, or returned May be on MAR, flowsheets in patient chart For CS stored in lockbox or non-automated storage location, requires manual process tovalidate logged inventory against actual, typically every shift or at least daily Patient specific dispensing of CS, ensure delivery and administration records returnedand reconciled Nursing controlled drug records One sheet lists all drugs (example page 18.11) End of shift counts (confirmation bias) Patient specific administration record - nurse records each dose administered, waste,and remaining quantity. Completed sheet and any non-administered CS returned topharmacy. Retain with CS records.18.5
Monitor anesthesia records for complete documentation, trend by user, partner withanesthesia and OR leadership If possible, incorporate data analytics software for monitoring. Otherwise, this is amanual process Trend discrepancies by unit and user level (inventory and waste transactiondiscrepancies) Compare user CS transactions to users on same unit, assess for outliers (utilizestandard deviation) Pharmacy – internal monitoring Vault blind count inventory on scheduled basis; completed by two techs Biennial inventory of all schedule II-V (every two years) Restock audit report to confirm quantity of CS dispensed from vault is received into ADCon unit (system alerts after defined period of time, giving tech time to deliver) Monitor waste documentation (used for compounding) Consider cameras or other security measures Monitor for diversion and tampering, inspect tamper seals, consider refractive index Don’t be complacent or too trusting; diverters typically don’t look the partPrescribing considerations Interns, residents, housestaff may prescribe CS under the registration of the hospital.Hospital must maintain list of internal codes for agent prescribers Example: ARNPs can write orders for CS for inpatients within their scope of practice and pursuantto medical staff protocols Florida law does not allow ARNPs to prescribe controlled substances. All prescriptions forcontrolled substances must be written and signed by a licensed physician. PAs cannot prescribe or dispense CS; however, a PA can order CS for inpatientsA supervising physician may delegate to a PA the authority to order medications for thesupervising physician’s patient during his/her care in a facility licensed under Chapter395, Florida Statutes, notwithstanding any provisions in Chapter 465 or 893, FloridaStatutes. An order is not considered a prescription in this instance.Theft or Significant Loss Notify local DEA Diversion Field Office within one business day of discovery of theft orsignificant loss Notify law enforcement (recommended but not required by federal law) Complete DEA Form 106 (available as online form; can amend)18.6
Notify Board of Pharmacy within 1 business day after discovery of theft or loss (465.022) TJC MM.01.01.03 “abuses and losses are reported to the Director of Pharmacy and the ChiefExecutive Officer” Collaborate with HR and Occupational Health. Be familiar with HR policies related to drugfree workplace, for cause drug testing, management of suspected diversion, coordinationwith PRN (Professionals Resource Network), etc. Collaborate with nursing and anesthesia – recognition of impaired professionalPolicies and Procedures Consider policies for: Inventory management including ordering and receiving, tracking, scheduling ofinventory counts Scope of services (especially if multiple DEA licenses, offsite offices, EDs or ambulatorysurgery centers – who is responsible for what?) Access levels of personnel Surveillance and monitoring Discrepancy resolution Handling and documentation of waste Disposal and destructionRecordkeeping All records including purchasing, receiving, storage, distribution, dispensing, disposal CS records must be maintained for 4 years Schedule II separate from III-V Executed 222 forms separate from other documents (although can attach to matchedinvoice) Records to be maintained DEA 222 (executed and unexecuted forms) or CSOS Power of Attorney authorization to sign forms Receipts and invoices for schedule III, IV and V controlled substances All inventory records including the initial and biennial inventories, dated as ofbeginning or close of business Records of controlled substances distributed or dispensed DEA 106 Inventory of Drugs Surrendered for disposal (DEA form-41 or Reverse Distributor) Records of transfers of controlled substances between pharmacies DEA registration certificate Other issues Updated record of doctor DEA numbers Prescription pad control18.7
Medicare COP standards:42 CFR Ch. IV (10–1–11 Edition)§482.25(a)(3) - Current and accurate records must be kept of the receipt and disposition of allscheduled drugs.18.8
SAMPLE POWER OF ATTORNEY FORMPower of Attorney for DEA Forms 222 and Electronic Orders(Name of registrant)(Address of registrant)(DEA registration number)I, (name of person granting power), the undersigned, who am authorizedto sign the current application for registration of the above-named registrant under the ControlledSubstances Act or Controlled Substances Import and Export Act, have made, constituted, andappointed, and by these presents, do make, constitute, and appoint(name of attorney-in-fact), my true and lawful attorney for me in my name, place, and stead, toexecute applications for Forms 222 and to sign orders for Schedule I and II controlled substances,whether these orders be on Form 222 or electronic, in accordance with 21 U.S.C. 828 and Part1305 of Title 21 of the Code of Federal Regulations. I hereby ratify and confirm all that saidattorney must lawfully do or cause to be done by virtue hereof.(Signature of person granting power)I, (name of attorney-in-fact), hereby affirm that I am the person namedherein as attorney-in-fact and that the signature affixed hereto is my signature.(signature of attorney-in-fact)Witnesses:1.2.Signed and dated on the day of , (year), at .Notice of RevocationThe foregoing power of attorney is hereby revoked by the undersigned, who is authorized to signthe current application for registration of the above-named registrant under the ControlledSubstances Act or the Controlled Substances Import and Export Act. Written notice of thisrevocation has been given to the attorney-in-fact this same day.(Signature of person revoking power)Witnesses:1.2.Signed and dated on the day of , (year), at .18.9
SAMPLE SURVEILLANCE TABLEREPORT AILYCONTROLLED SUBSTANCEPHARMACY TECHNICIAN (CST)This report identifies any discrepancies noted bynursing that have not been resolved.Discrepancies that have not been resolved within 24hours of their inception will be communicated to theappropriate nurse manager. The Controlled SubstanceTechnician will run additional Omnicell reports, gatherthe necessary information, and discuss the resolutionof the discrepancy with the Pharmacy manager for theVault area.When needed the Assistant Director, IP PharmacyOperations will arrange to discuss unresolveddiscrepancies with the appropriate nursing manager.Discrepancies that have not been resolved will berecorded by the Controlled Substance Technician. Amonthly report by nursing area will be generated anddistributed to the nursing and pharmacy managers.RESOLVED DISCREPANCIESDAILYCSTThis report is used to monitor the resolution ofcontrolled substance discrepancies. The analysisshould include appropriateness of resolution reasonand the identification of patterns related to individualusers.Acceptable resolutions will be filed.VAULT INVENTORYDAILYCSTVAULT INVENTORYWEEKLYCSTDISPENSE/RETURN/WASTEDAILYAS NEEDEDRESTOCK AUDITDAILYCSTNON-VAULT SUPPLEMENTALRESTOCKDAILYCSTEXPIRED MED REPORTDAILYCSTEMPTY DRAWER RETURNEND OF CYCLECABINET CYCLE COUNTWEEKLYDAILYQUARTERLYCST AND AUTOMATION TECHSCSTCST AND AUTOMATION TECHS18.10Unacceptable resolutions will investigated jointly bynursing and pharmacy management and correctiveBlind, manual count of all items involved in any SecureVault transaction since previous End of Cycle ReportBlind, manual count of ALL Secure Vault inventoryitems.DEA requirement to document transactions for allscheduled medications. Report generatedautomatically and stored electronically, available asreference if needed.DESTOCK/RESTOCK matching: identify discrepanciesbetween controlled substances ISSUED from SecureVault and RESTOCKED at the designated cabinet.Two-Fold purpose: 1. Evaluate cabinet inventory (parsand reorder points) 2. DESTOCK/RESTOCK matchingfor off hours dispensing.Monitor expired controlled substances stocked inADCs. Report looks for past one year and 14 days inadvance.The cycle count assignments will be rotated to ensureno single person counts the same cabinet twice in 2consecutive months.
MedicationPatient NameTimeDosageGIVENWASTED18.11RECORD ALL WASTE WITH SIGNATURE ON BACK OF FORMMethadone 10 MG tabLorazepam 1 MG tabLorazepam 0.5 MG tabSignature #2Hydromorphone 2 MG tabHydrocodone/Acet 7.5-500 MG tabHydrocodone/Acet 5/500MG tabHydrocodone/APAP 2.5/500 MG tabHydrocodone/Acet 10/500 MG tabControlled Drug Record Proof of Use FormORAL CONTROLLED DRUGSDateBALANCE from previous Signature #1Diazepam 5 MG tabClonazepam 1MG tabClonazepam 0.5 MG tabAlprazolam 0.5 MG tabsheet verified by:Alprazolam 0.25 MG tabUnitNurse SignatureSAMPLE FORMNOTE: Top of box is the transaction amount.Bottom of box is the final count.Page of
18.12
18.13
18.14
18.15
18.16
18.17
NURSING HOMEControlled Drugs in the Nursing HomeI. Regulatory Overview1. Board of Pharmacy Regulations 64BB16-28.301 Destruction of Controlled Substances –Class I (Institutional Pharmacies – Nursing Homes)2. Controlled Substances Act 10D-463. Nursing Home Regulations 59A-4.112 Pharmacy Services(1) The consultant pharmacist shall establish a system to accurately record the receipt anddisposition of all controlled drugs in sufficient detail to enable an accuratereconciliation.(2) The pharmacist shall determine that drug records are in order and that an account of allcontrolled drugs is maintained and periodically reconciled.(3) All controlled substances shall be disposed of in accordance with State and federal laws.All non-controlled substances may be destroyed in accordance with the facility’spolicies and procedures. Records of the disposition of all substances shall be maintainedin sufficient detail to enable an accurate reconciliation.4. Federal Survey ManualF431 MEDICATION STORAGE, LABELING, AND CONTROLLED SUBSTANCES GUIDANCE(Excerpts)INTENT (F431) 42 CFR 483.60(b)(2)(3)(d)(e)The intent of this requirement is that the facility has in place a functioning medication system that includes theservices of a licensed pharmacist and provides for: Safe and secure storage (including proper temperature controls and limited access) and safe handling(including disposition) of all med
Cabinet access restricted to authorized users Can restrict based on role (respiratory therapists can access cabinet and specific bins but cannot access CS or other drugs they don’t administer) Maintains perpetual inventory and transaction record, provides discrepancy notificatio