Transcription
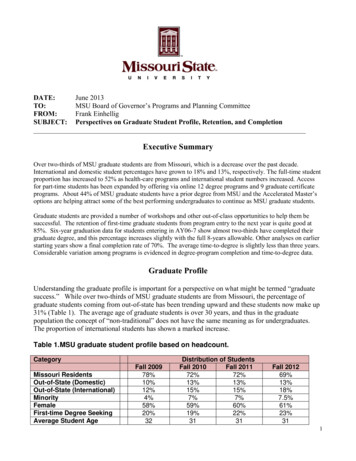
LegendWorkbook LegendWorksheet NameCost Average SummaryDeleware ‐ TennesseeDefinitionPopulation Grouping2/17/2012DescriptionProvides average costs for start‐up, engagement and maintenance phases of ELR,grouped by population size for the nine participating states.State Profiles that capture information about each state's ELR architecture andsystem functionProvides definitions of each aspect of architecture and system function capturedin the state profiles.Worksheet shows assignment of population to small, medium, and large forpurposes of the Cost Average Summary graphCSTE/CDC ELR Task ForceResource and Capacity Assessment Workgroup1
Cost Average SummaryGrouped by PopulationSize (in percentile)0 ‐ 50 percentile51 ‐ 75 percentile 75 percentileELR PhaseStart Up (n 4)Engagement (n 3)Maintenance (n 3)Start Up (n 2)Engagement (n 2)Maintenance (n 2)Start Up (n 3)Engagement (n 3)Maintenance (n 3)Average 297,375 221,000 221,500 405,000 338,250 233,250 600,000 633,500 458,000Average Estimated Cost for State Resource Grouped by Population Sizein Percentile per ELR Phase (N 9) 700,000 600,000 500,000 400,000 300,000 200,000 100,000 0Start Up (n 4)Engagement(n 3)0 ‐ 50 percentile2/17/2012Maintenance(n 3)Start Up (n 2)Engagement(n 2)Maintenance(n 2)Start Up (n 3)51 ‐ 75 percentileCSTE/CDC ELR Task ForceResource and Capacity Assessment WorkgroupEngagement(n 3)Maintenance(n 3) 75 percentile2
Deleware State ProfileDelawareIn Delaware, electronic laboratory reporting (ELR) is primarily supported by six people, among them representing threeFTEs. ELR is received and processed The majority of ELR reports received by the state are consumed in the DelawareElectronic Reporting and Surveillance System (DERSS). The receipt of ELR for positive test results and test orders for statereportable conditions are sent to the DERSS and to Enhanced HIV/AIDS Reporting System (eHARS). Currently, ELR isreceived in HL7 2.3.1 and v2.3z format.System FunctionsArchitectureELR System CharacteristicThere are two EAIs used. The state uses a NEDSS‐based legacy HL7integration engine (state developed and coupled within DERSS), as wellas the CDC funded COTS product, Orion Health’s Rhapsody IntegrationEngine (Rhapsody). Rhapsody is installed as a stand‐alone system.Along with Rhapsody, the state also uses NEDSS Message SubscriptionService (MSS) to process and route incoming ELR of positive test resultsEnterprise Application Integrationfor state reportable diseases and PHINMS for message transport .(EAI) SystemIntegration to Standards System (e.g. N/AELR is routed to the appropriate disease surveillance informationsystems: DERSS, STD/MIS (future; post‐receipt of new version ofSTD/MIS), eHARS, Lead / HHLPSS, the Delaware Cancer Registry, DelVAX(Delaware immunization registry), and the electronic medical recordsystem (EMR). NOTE: Some systems are in test versus productionList surveillanceg systems receiving ELR status.Compatible system (e.g. programassignment)ELR received in DERSS serves as management tool and use to identify co‐morbidityManagement of Local CodesManages local codes in DERSS. At this time, it is unknown if Rhapsodyalso stores local codes for other conditions.Management of Mapping tostandardsManages mappings in legacy HL7 integration engine in DERSS.Web‐based servicesRhapsody 4.1 (TEST environment till end‐October) provides web‐basedservices for content validation, content translation, and subscription.Currentlly in production with Rhapsody v3.4.HL7 Message ValidationRhapsody provides structural validation and legacy HL7 integrationengine in DERSS provides content validation.RoutingManagement of routes is configured in Rhapsody to send ELR messagesto the appropriate surveillance application.Alerting (email, SMS)Reporting2/17/2012DescriptionNot currently activeLegacy HL7 integration engine produces daily reports of ELR systemstatus and performance. Use DERSS to export data for epidemiologicalanalyses.CSTE/CDC ELR Task ForceResource Capacity Assessment Workgroup3
Deleware State ProfileVolume of CasesPopulation Size (Range)1 ‐ 5,000,000With Lab InitiatedInfoby ELR80‐85% 75 ‐ 80%100%80‐85%100%0***85%0****8%3%100%0Total number of Cases by Condition TypeGeneral Communicable DiseaseHIV/AIDSSexual Transmitted DiseaseTuberculosisCancerEnvironment HealthCases*8,4001358,600206,60015Reporting EntitiesEstimated Number in JurisdictionNumber currently in TEST with ELRNumber currently in production with ELREligible HospitalsIndependentLabsPublic HealthLabs8606041N/A1*Counts are approximate, and represent a 12‐month period.**Does not include chronic hepatitis C***Electronic results are transmitted to us by multiple labs, but are notyet receivable by the STD program’s system (STDMIS). Upon availability ofa newer version of STDMIS, it is anticipated these results will be captured.****Virtually always initiated by a call / faxed report of possible /suspected case, with follow‐on report of laboratory resultsEngagement Process (optional)FYI: We are in the midst of working through health information exchange (HIE) / non‐HIE transmissions; no estimatesavailable at this time. [Need to verify HIE flow]Messaging FunctionEligible HospitalIndependent LabsRange of Hours Spent Range of Hours SpentPublic Health LabRange of Hours SpentHL7 Message (structure andContent) ValidationManagement of Local CodesManagement of Mapping tostandardsRouting2/17/2012CSTE/CDC ELR Task ForceResource Capacity Assessment Workgroup4
Florida State profileFloridaIn Florida, electronic laboratory reports received by the state are processed and stored in a central ELRdatabase. Results of interest (those meeting the case definition or identified as needing further investigation)are pulled from the ELR database and consumed in the Merlin electronic disease surveillance system forgeneral communicable diseases, and heavy metals (i.e. lead, mercury, etc). All results received in the centralELR database are accessible via user driven search queries from a search screen within the Merlin surveillanceapplication ‐ for example both positive and negative test results for specimens tested at the state publichealth lab (outbreak specimens, etc) can be searched. HL 2.5.1, 2.3.1, 2.3.X, flat files.ArchitectureELR System Characteristic2/17/2012DescriptionFlorida uses a COTS product, Cloverleaf, to process andtranslate incoming ELR messages for state reportableEnterprise Application Integration (EAI) conditions. ELR data inserted and stored in a central ELRSystemdatabase.N/A. FLDOH receives and process all results sent to theintegration broker (Cloverleaf) and populates the centralELR database. The sending facility and application willsend either local or LOINC codes. Translation toIntegration to Standards System (e.g. standards acceptable to Florida are performed by theCloverleaf interface engine.VADS, RELMA, UMLS)ELR is pulled out of the central ELR database by theappropriate disease surveillance information system:Merlin, Prism (STD); each system identifies and consumesrecords of interest in different ways according to theapplication design; Merlin functions are completlyautomated with results accessible by epidemiologistsresponsible for conducting case investigations in each ofthe 67 counties as soon as the results are received by thedepartmentList surveillance systems receiving ELRAreas of integration with NEDSSCompatible system (e.g. programassignment)N/AManagement of Local CodesLocal codes are managed from within the Merlinapplication in the form of "filters;" Filter rules are set upper laboratory sending facility/application and are appliedto local or standard codes (LOINC ) depending on thefacility ability to send standard or local codes.Management of Mapping to standardsThe Cloverleaf software is used to transform incoming ELRmessages into a format used to insert the data into theCentral ELR database. Most incoming ELR messages areconverted to HL7 2.3.1.CSTE/CDC ELR Task ForceResource and Capacity Assessment Workgroup5
Florida State profileHL7 Message ValidationCloverleaf does that Content validation, contenttranslation, and subscription; web‐based services are notutilizedCloverleaf validates every HL7 message to ensure it hasthe proper message structure and segments and certainrequired fields (date of birth, patient name, specimen ID,accession number)RoutingResults are populated into the centeral ELR database. Thesurveillance application, Merlin, maintains filters bylaboratory, test and specimen that are used to identifyrecords of intersts and "pull" them into the surveillanceapplication; the process is completely automated andrecords of interest are immediately available to diseaseinvestigators in each of the 67 counties via county specificMerlin ELR Task Lists as soon as results are popluated intothe ELR databaseAlerting (email, SMS)From within Merlin, laboratory disease specific emailalerts with results of interest can be sent to any user ofthe Merlin system; Merlin alerts are maintained on userprofiles; Cloverleaf ‐ alerts sent out if a laboratory facilitydoes not send in any results for the dayReportingProduce daily, weekly, monthly, and yearly reports of ELRsystem status, performance, and/or trends; Cloverleaf ‐produces reports of daily volume, status of types ofresults sent out; monthly report on data content toensure overall content remains at an acceptable levelSystem FunctionsWeb‐based servicesVolume of CasesPopulation Size (Range)Total number of Cases by Condition TypeGeneral Communicable DiseaseHIV/AIDSSexual Transmitted DiseaseTuberculosisCancer (2008, Newly diagnosed cases)Environment HealthReporting EntitiesEstimated Number in JurisdictionNumber currently in production with ELR2/17/201215,000,000 ‐ 20,000,000With Lab InitiatedInfoby ELRCases*51000 95%8700 95%99000 95%850 95%105200IndependentEligible Hospitals Labs22825CSTE/CDC ELR Task ForceResource and Capacity Assessment Workgroup44615Public HealthLabs556
Florida State profileEngagement Process (optional)Messaging FunctionHL7 Message (structure andContent) ValidationManagement of Local CodesManagement of Mapping tostandardsRouting2/17/2012Eligible HospitalRange of Hours SpentIndependent LabsPublic Health LabRange of Hours Spent Range of Hours SpentCSTE/CDC ELR Task ForceResource and Capacity Assessment Workgroup7
Idaho State profileIdahoIn Idaho, electronic laboratory reporting (ELR) is primarily supported by 1.5 number of FTE(s). All ELR reportsreceived by the state are consumed in the NEDSS Base System (NBS). The receipt of ELR for positive testresults for state reportable diseases is sent to the NBS via Rhapsody. Currently, ELR is received in HL7 2.3.1format.ArchitectureELR System CharacteristicThe state uses CDC funded COTS product, Orion Health’sRhapsody Integration Engine (Rhapsody) and NEDSSMessage Subscription Service (MSS) to process incomingEnterprise Application Integration (EAI) receipt of ELR for positive test results for state reportableconditions. Rhapsody is installed as a stand‐alone system.SystemIntegration to Standards System (e.g.VADS, RELMA, UMLS)Only with PHIN VADS via MSS.List surveillance systems receiving ELRNEDSS Base System (NBS) and Enhanced HIV/AIDSReporting System (eHARS).Areas of integration with NEDSSCompatible system (e.g. programassignment)Management of Mapping to standardsN/A. Rhapsody/MSS is installed as a stand alone systemand ELR is routed to the appropriate disease surveillanceinformation system.Manages local codes in NEDSS Message SubscriptionServiceManages mappings in NEDSS Message SubscriptionServiceWeb‐based servicesRhapsody provides web‐based services for Contentvalidation, content translation, and subscriptionHL7 Message ValidationRhapsody and MSS provide structural and contentvalidationRoutingManagement of routes are configured in Rhapsody tosend ELR messages to the appropriate surveillanceapplicationSystem FunctionsManagement of Local CodesAlerting (email, SMS)Reporting2/17/2012DescriptionWithin Rhapsody, the system can generate email alerts inthe event of a receipt of immediate reportable condition.Use alerting extensively in ELR process. Whenever an ELRmessage is received that fails validation, is unrecognized,is missing standard codes, has unrecognized specimensources, etc. then Rhapsody sends Project Manager anemail.Produce daily, weekly, monthly, and yearly reports of ELRsystem status, performance, and/or trends using the NBSto export data for analysisCSTE/CDC ELR Task ForceResource and Capacity Assessment Workgroup8
Idaho State profileVolume of CasesPopulation Size (Range)1 ‐ 5,000,000Total number of Cases by Condition TypeGeneral Communicable DiseaseHIV/AIDSSexual Transmitted DiseaseTuberculosisCancerEnvironment HealthWith LabInfo3,203Initiatedby ELR7924,377150Eligible HospitalsIndependentLabsPublic HealthLabs52424711Cases*3,598834,3771581Reporting EntitiesEstimated Number in JurisdictionNumber currently in production with ELREngagement Process (optional)Messaging FunctionHL7 Message (structure andContent) ValidationManagement of Local CodesManagement of Mapping tostandardsRoutingEligible HospitalRange of Hours SpentIndependent LabsPublic Health LabRange of Hours Spent Range of Hours Spent100 ‐ 400050 ‐ 2500450300 ‐ 6050 ‐ 1500 ‐ 105 ‐ 10050400Message Validation – this includes validating message structure and content, working with the labto try and adjust format and especially content. It also includes the parallel validation where wematch all incoming ELR’s to manual reports for 90 days and follow‐up on any discrepancies. Thisoften last longer than 90 days as we require 90 days error free. The PHL hours also include the1 validation of the CDC reporting messages such as PHLIS, PHLIP and LRN.Local codes are allocated by the Labs so the only Lab that worked on local codes is the State Lab.This however was on the Lab side and not really on the message side. If John is only looking at the2 messages once they come out of the LIMS system then it should be zero as well.I assumed that this was the mapping of local codes to standard codes. Many of the Labs do thisthemselves but for some Labs we have had to either ask for changes or in some cases do the3 complete mapping for them.2/17/2012CSTE/CDC ELR Task ForceResource and Capacity Assessment Workgroup9
Idaho State profileUnder the routing category I included the changes we make in Rhapsody to reformat/excludemessages from Labs that cannot meet our exclusion criteria, labs that mix reportable/non‐reportable results and the Labs that send comments as results or results as comments. For theState Lab, I also include all the routing and reformatting necessary to include the PHLIS, PHLIP and4 LRN reporting.2/17/2012CSTE/CDC ELR Task ForceResource and Capacity Assessment Workgroup10
Kansas State ProfileKansasIn Kansas, electronic laboratory reporting (ELR) is primarily supported by 3.0 FTE(s). The majority of futureELR reports received by the state will be consumed in the Kansas electronic disease surveillance system,TriSano. The receipt of ELR for positive test results for state reportable diseases will be sent to TriSano viaRhapsody. ELR will be received in HL7 2.5.1 format.ELR System CharacteristicDescriptionArchitectureEngine (Rhapsody), installed as a standalone tool, and willEnterprise Application Integration (EAI) be used to processes incoming receipt of ELR for stateSystemreportable diseases.Integration to Standards System (e.g.VADS, RELMA, UMLS)List surveillance systems receiving ELRAreas of integration with NEDSSCompatible system (e.g. programassignment)Management of Local CodesManagement of Mapping to standardsSystem FunctionsWeb‐based servicesHL7 Message ValidationRoutingAlerting (email, SMS)Reporting2/17/2012NoN/A. When the ELR feed is active it will be routed to theTriSano for general communicable disease, TB, and STD. Inthe future, Lead and HIV results will be routed to theappropriate system.Rhapsody is installed as a stand‐alone system. ELR will berouted to the appropriate disease surveillanceinformation system: TriSano, eHARS, leadA decision has not been made.Everything that comes into Rhapsody will be mapped toHL7 2.5.1 standardsNot known at this timeIt is expected that Rhapsody will provide structuralvalidation and the programs will conduct contentvalidation.Management of routes are configured in Rhapsody tosend ELR messages to the appropriate surveillanceapplicationWithin Trisano, the system will be able to generate emailalerts in the event of a receipt of immediate reportableconditionOnce ELR is in production, the system will produce daily,weekly, monthly, and yearly reports of ELR system status,performance, and/or trends using the Trisano AnalysisVisualization and Reporting toolCSTE/CDC ELR Task ForceResource and Capacity Assessment Workgroup11
Kansas State ProfileVolume of CasesPopulation Size (Range)1 ‐ 5,000,000Total number of Cases by Condition TypeGeneral Communicable DiseaseHIV/AIDSSexual Transmitted DiseaseTuberculosisCancerEnvironment HealthCases8477186117034541With LabInfo71174541Initiatedby ELR0000IndependentPublic HealthEligible Hospitals LabsLabsReporting EntitiesEstimated Number in Jurisdiction126*181Number currently in production with ELR000* This is the number of hospitals in Kansas; the number of eligible hospitals for ELR is not known atthis timeEngagement Process (optional)**Eligible HospitalRange of Hours SpentIndependent LabsPublic Health LabRange of Hours Spent Range of Hours SpentMessaging FunctionHL7 Message (structure andContent) ValidationManagement of Local CodesManagement of Mapping tostandardsRouting**Kansas cannot calculate this information at this time, as we have not been through the engagement processyet.2/17/2012CSTE/CDC ELR Task ForceResource and Capacity Assessment Workgroup12
Nebraska State ProfileNebraskaIn Nebraska, all electronic laboratory reporting (ELR) reports received by the state are consumed in the NEDSSBase System (NBS). The receipt of ELR for positive and negative test results for state reportable conditions aresent to the NBS via Rhapsody. Currently, ELR is received in HL7 2.3.1 formatELR System CharacteristicDescriptionArchitectureThe state uses CDC funded COTS product, Orion Health’sRhapsody Integration Engine (Rhapsody) to processEnterprise Application Integration (EAI) incoming receipt of ELR for state reportable conditions.Rhapsody is installed as a stand‐alone system.SystemIntegration to Standards System (e.g.VADS, RELMA, UMLS)Only with PHIN VADS.List surveillance systems receiving ELRAreas of integration with NEDSSCompatible system (e.g. programassignment)Management of Local CodesELR is routed to the appropriate disease surveillanceinformation system: NBS. STD/MIS, Enhanced HIV/AIDSReporting System, and STELLAR (lead) receive an ELRextract.STD/MIS and Enhanced HIV/AIDS Reporting System(eHARS). All ELR, including lead. The method of exportingis different for each receiving surveillance application.? [This is unknown at this time]System FunctionsManagement of Mapping to standards2/17/2012Web‐based services? [This is unknown at this time]Rhapsody provides web‐based services for Contentvalidation, content translation, and subscriptionHL7 Message ValidationRhapsody provides structural validation. Programconducts content validation.RoutingManagement of routes are configured in Rhapsody tosend ELR messages to the appropriate surveillanceapplicationAlerting (email, SMS)Within NBS, the system can generate email alerts in theevent of a receipt of immediate reportable conditionReportingProduce daily, weekly, monthly, and yearly reports of ELRsystem status, performance, and/or trends using the NBSReporting Database to export data for analysisCSTE/CDC ELR Task ForceResource and Capacity Assessment Workgroup13
Nebraska State ProfileVolume of CasesPopulation Size (Range)1 ‐ 5,000,000With LabInfoCases*5,4564,505110,33431Total number of Cases by Condition TypeGeneral Communicable DiseaseHIV/AIDSSexual Transmitted DiseaseTubercul
integration broker (Cloverleaf) and populates the central ELR database. The sending facility and application will send either local or LOINC codes. Translation to standards acceptable to Florida are performed by the Cloverleaf interface engine