Transcription
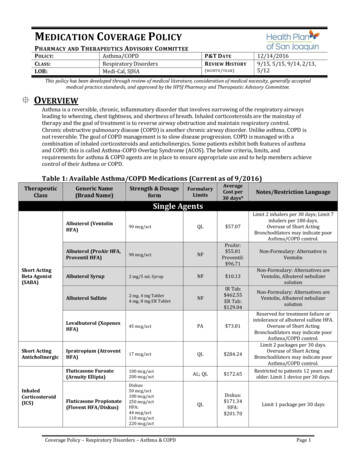
MEDICATION COVERAGE POLICYPHARMACY AND THERAPEUTICS A DVISORY C OMMITTEEP OLICY:C LASS:LOB:Asthma/COPDRespiratory DisordersMedi-Cal, SJHAP&T D ATER EVIEW HISTORY(MONTH / YEAR )12/14/20169/15, 5/15, 9/14, 2/13,5/12This policy has been developed through review of medical literature, consideration of medical necessity, generally acceptedmedical practice standards, and approved by the HPSJ Pharmacy and Therapeutic Advisory Committee. OVERVIEWAsthma is a reversible, chronic, inflammatory disorder that involves narrowing of the respiratory airwaysleading to wheezing, chest tightness, and shortness of breath. Inhaled corticosteroids are the mainstay oftherapy and the goal of treatment is to reverse airway obstruction and maintain respiratory control.Chronic obstructive pulmonary disease (COPD) is another chronic airway disorder. Unlike asthma, COPD isnot reversible. The goal of COPD management is to slow disease progression. COPD is managed with acombination of inhaled corticosteroids and anticholinergics. Some patients exhibit both features of asthmaand COPD; this is called Asthma-COPD Overlap Syndrome (ACOS). The below criteria, limits, andrequirements for asthma & COPD agents are in place to ensure appropriate use and to help members achievecontrol of their Asthma or COPD.Table 1: Available Asthma/COPD Medications (Current as of 9/2016)TherapeuticClassGeneric Name(Brand Name)Strength & DosageformAverageCost per30 days*Notes/Restriction LanguageQL 57.07Limit 2 inhalers per 30 days; Limit 7inhalers per 180 days.Overuse of Short ActingBronchodilators may indicate poorAsthma/COPD control.Non-Formulary: Alternative isVentolinFormularyLimitsSingle AgentsAlbuterol (VentolinHFA)Short ActingBeta Agonist(SABA)Short ActingAnticholinergicInhaledCorticosteroid(ICS)90 mcg/actAlbuterol (ProAir HFA,Proventil HFA)90 mcg/actNFProAir: 55.81Proventil: 96.71Albuterol Syrup2 mg/5 mL SyrupNF 10.13Non-Formulary: Alternatives areVentolin, Albuterol nebulizersolutionAlbuterol Sulfate2 mg, 4 mg Tablet4 mg, 8 mg ER TabletNFIR Tab: 462.55ER Tab: 129.04Non-Formulary: Alternatives areVentolin, Albuterol nebulizersolutionLevalbuterol (XopenexHFA)45 mcg/actPA 73.81Ipratropium (AtroventHFA)17 mcg/actQL 284.24Fluticasone Furoate(Arnuity Ellipta)100 mcg/act200 mcg/actAL; QL 172.65Fluticasone Propionate(Flovent HFA/Diskus)Diskus:50 mcg/act100 mcg/act250 mcg/actHFA:44 mcg/act110 mcg/act220 mcg/actQLDiskus: 171.34HFA: 201.70Coverage Policy – Respiratory Disorders – Asthma & COPDReserved for treatment failure orintolerance of albuterol sulfate HFA.Overuse of Short ActingBronchodilators may indicate poorAsthma/COPD control.Limit 2 packages per 30 days.Overuse of Short ActingBronchodilators may indicate poorAsthma/COPD control.Restricted to patients 12 years andolder. Limit 1 device per 30 days.Limit 1 package per 30 daysPage 1
TherapeuticClassLong ActingBeta Agonist(LABA)Long 5-LipoxygenaseInhibitorGeneric Name(Brand Name)Strength & DosageformMometasone Furoate(Asmanex Twisthaler)110 mcg/act (30 doses)220 mcg/act (30, 60, or120 doses)Mometasone Furoate(Asmanex HFA)BeclomethasoneDipropionate (Qvar)FormularyLimitsAverageCost per30 days*Notes/Restriction LanguageLimit 1 package per 30 days.110 mcg: Restricted to patientsunder the age of 12.Non-Formulary: Alternatives arePulmicort Flexhaler, AsmanexTwisthaler, Qvar, FloventHFA/DiskusAL (110mcg); QL 195.61100 mcg/act200 mcg/actNF 233.3040 mcg/act80 mcg/actQL 178.76Limit 1 package per 30 daysBudesonide (PulmicortFlexhaler)90 mcg/actNF 159.94Non-Formulary: Alternatives areFlovent HFA 44 mcg, Flovent Diskus50 mcg, Asmanex Twisthaler 110mcg, Qvar 40 mcgBudesonide (PulmicortFlexhaler)180 mcg/actQL 192.23Limit 1 package per 30 daysFlunisolide (Aerospan)80 mcg/actNF 235.31Ciclesonide (Alvesco)80 mcg/act160 mcg/actNF 186.6250 mcg/actNF 314.79ST; QL 246.66Salmeterol Xinafoate(Serevent Diskus)Formoterol Fumarate(Foradil)12 mcg InhalationCapsuleNon-Formulary: Alternatives arePulmicort Flexhaler, AsmanexTwisthaler, Qvar, FloventHFA/DiskusNon-Formulary: Alternatives arePulmicort Flexhaler, AsmanexTwisthaler, Qvar, FloventHFA/Diskus, Arnuity ElliptaNon-Formulary: Alternative isStriverdi RespimatConcurrent use of ICS is required.Limit 1 package per 30 days.Indacaterol Maleate(Arcapta Neohaler)75 mcg/actNF 256.33Non-Formulary: Alternative isStriverdi RespimatOlodaterolHydrochloride(Striverdi Respimat)2.5 mcg/actST; QL 201.79Concurrent use of ICS is required.Limit 1 package per month.Tiotropium Bromide(Spiriva)Handihaler:18 mcg InhalationCapsuleRespimat:2.5 mcg/actTiotropium Bromide(Spiriva Respimat)1.25mcg/actST 348.85Aclidinium Bromide(Tudorza Pressair)400 mcg/actPA; QL 290.32Umeclidinium Bromide(Incruse Ellipta)62.5 mcg/actNF 327.30Montelukast Sodium(Singulair)4 mg Oral Granules4 mg, 5 mg ChewableTablet10 mg TabletQL 12.39Limit 30 tablets per 30 daysZafirlukast (Accolate)10 mg, 20 mg TabletNF 98.93Non-Formulary: Alternative ismontelukastZileuton (Zyflo, ZyfloCR)600 mg Tablet600 mg ER TabletNF 2,980.00Indicated for Asthma onlyCoverage Policy – Respiratory Disorders – Asthma & COPDPA; QL(Respimat)Handihaler: 332.65Respimat: 340.95Documentation of diagnosis ofGOLD Grade II COPD is required forapproval.Respimat: Limit 1 package per 30days.Step therapy to Montelukast ANDone of the following: Symbicort (160mcg/4.5 mcg), Advair (500 mcg/50mcg), or Dulera (200 mcg/5 mcg)within the last 30 days.Documentation of diagnosis of GOLDGrade II COPD is required forapproval. Limit 1 package per 30days.Non-Formulary: Alternatives areSpiriva Handihaler, Spiriva Respimat2.5 mcg, TudorzaPage 2
hibitor,NonselectivePDE-4 InhibitorMonoclonalAntibody, AntiAsthmaticGeneric Name(Brand Name)Strength & DosageformFormularyLimitsAverageCost per30 days*Notes/Restriction Language80mg/15mL OralElixir/Solution100 mg, 200 mg, 300 mg,ER Cap (Theo-24)100 mg, 200 mg, 300 mgER Tab (Theochron, 12hr)400 mg, 600 mg ER Tab(24-hr)450 mg ER Tab(Theochron, 12-hr)--Theophylline (Theo24)400 mg ER CapNF 131.51Theophylline400 mg, 800 mg IVSolutionNF 21.02Roflumilast (Daliresp)500 mcg TabletPA; ST 278.08Omalizumab (Xolair)150 mg VialPA 2,014.88Mepolizumab (Nucala)100 mg VialPA 3,090.00Reserved for patients withpoorly controlled, severeeosinophilic asthmaReslizumab (Cinqair)100 mg/10 mL IVSolutionNF 100.20per vialIndicated for Asthma only. Dose isweight-dependent (3 mg/kg).Theophylline (Theo24, Elixophyllin,Theochron)Elixophyllin: 378.39Theo-24: 116.07Theochron: 17.24Narrow therapeutic window. Shouldbe reserved as last line therapy.24-hr tabs(400 mg,600 mg): 42.57Non-Formulary: Alternative istheophylline 400 mg ER tabletIndicated for COPD only.Reserved for GOLD Grade III COPDin patients compliant on ICS/LABAand Spiriva/Tudorza.Reserved for inadequate asthmacontrol or uncontrolled chronicidiopathic urticariaCombination AgentsShort ActingCombinationLong ActingCombinationIpratropium/Albuterol(Combivent e/Formoterol (Dulera)20 mcg-100 mcgQL 312.42Limit 1 package per 30 days. Shouldnot be used with Tiotropium.80 mcg-4.5mcg160 mcg-4.5 mcgQL 277.37Limit 1 package per 30 daysQL 265.98Limit 1 package per 30 daysFluticasone/Salmeterol (AdvairDiskus or HFA)Diskus:100 mcg-50 mcg250 mcg-50 mcg500 mcg-50 mcgHFA:45 mcg-21mcg115 mcg-21mcg230 mcg-21 mcgQLDiskus: 337.11HFA: 340.58Limit 1 package per 30 daysFluticasone/Vilanterol(Breo Ellipta)100 mcg-25 mcg200 mcg-25 mcgNF 272.74Tiotropium/Otodaterol (StioltoRespimat)2.5 mcg-2.5 mcgPA; QL 318.4962.5 mcg-25 mcgNF 378.8227.5 mcg-15.6 mcgNF 357.379 mcg-4.8 mcgNF 378.82Umeclidinium/Vilanterol (AnoroEllipta)Glycopyrrolate/Indacaterol (UtibronNeohaler)Glycopyrrolate/Formoterol (BevespiAerosphere)100 mcg-5mcg200 mcg-5mcgCoverage Policy – Respiratory Disorders – Asthma & COPDNon-Formulary: Alternatives includeAdvair, Symbicort, Dulera,CombiventReserved for patients with at leastGrade II (moderate) COPDconfirmed by PFTs. Limit 1 inhalerper 30 days.Non-Formulary: Alternatives includeAdvair, Symbicort, Dulera,Combivent, Stiolto RespimatNon-Formulary: Alternatives includeAdvair, Symbicort, Dulera,Combivent, Stiolto RespimatNon-Formulary: Alternatives includeAdvair, Symbicort, Dulera,Combivent, Stiolto RespimatPage 3
TherapeuticClassGeneric Name(Brand Name)Strength & DosageformFormularyLimitsAverageCost per30 days*Notes/Restriction LanguageSolution for NebulizationShort ActingBeta Agonist(SABA)Albuterol SulfateLevalbuterolHydrochloride0.63 mg/3 mL1.25 mg/3 mL2.5 mg/0.5 mL (0.083%)2.5 mg/3 mL5 mg/mL (0.5%)0.31 mg/3 mL0.63 mg/3 mL1.25 mg/3 mL1.25 mg/0.5 mLQL 19.85Limit 375 mL per 30 daysPA 292.60Reserved for patients withintolerance/contraindication toAlbuterolShort ActingAnticholinergicIpratropium Bromide0.02% NebulizationSolution-- 15.16Short ActingCombinationIpratropium/Albuterol (Duoneb)0.5 mg-3 mg(2.5 mgBase)/3 mLQL 33.08Limit 375 mL per 30 daysInhaledCorticosteroidBudesonide0.25 mg/2 mL0.5 mg/2 mL1 mg/2 mLAL; QL 297.51Limit 120 mL per 30 days.Restricted to members 4 years old.Long ActingBeta AgonistFormoterol )20 mcg/2 mLNF 666.42Non-Formulary: Formularyalternative is Serevent Diskus15 mcg/2 mlNF 612.39Non-Formulary: Formularyalternative is Serevent Diskus20 mg/2 mL------QL 17.89Limit 1 per lifetimeBubbles the Fish II Pedi MaskQL 1.65Optichamber Adult Mask (Large)QL 30.03Limit 1 per lifetime. Submit PA forlost/broken.Limit 2 per yearOptichamber Diamondwith maskLargeMediumSmallQL 28.51Limit 2 per yearVortex HoldingChamber with without maskChild Mask (Frog)Toddler Mask (Ladybug)QL 24.70Limit 2 per yearMast CellStabilizerCromolyn SodiumMedical EquipmentPeak Air Peak Flow MeterMask/SpacerFlow-VU:Aerochamber PlusFlow-VU/Plus Z-Stat/Z-stat Plus with maskLargeMediumSmallInspirachamber withmaskLargeMediumSmallLargeMediumSmall 45.81NFPlus Z-Stat: 34.20Z-Stat Plus:Non-Formulary: Alternatives areOptichamber, Vortex 28.50Easivent HoldingChamber with maskNebulizerNF 53.42Non-Formulary: Alternatives areOptichamber, VortexNF 57.78Non-Formulary: Alternatives areOptichamber, VortexQL--Limit 1 per lifetime.Max amount 100.PA Prior Authorization; QL Quantity Limit; AL Age Limit; NF Non-formulary*Cost/Rx based on HPSJ Medi-Cal utilization historical data from September 2015 through August 2016 EVALUATION CRITERIA FOR APPROVAL/EXCEPTION CONSIDERATIONBelow are the coverage criteria and required information for each agent. These coverage criteria have beenreviewed approved by the HPSJ Pharmacy & Therapeutics (P&T) Advisory Committee. For conditions notcovered under this Coverage Policy, HPSJ will make the determination based on Medical Necessity asdescribed in HSPJ Medical Review Guidelines (UM06).Coverage Policy – Respiratory Disorders – Asthma & COPDPage 4
Short Acting Beta AgonistsAlbuterol sulfate (Ventolin HFA, ProAir HFA, Proventil HFA, albuterol syrup, albuterol tablets), Levalbuteroltartrate (Xopenex HFA)Albuterol Sulfate (Ventolin HFA) Coverage Criteria: None Limits: 2 inhalers per 30 days; 7 inhalers per 180 days Required Information for Approval: N/A Other Notes: Ventolin HFA is the preferred Albuterol formulation. Use of more than 7 inhalers per180 day period may indicate uncontrolled asthma. Consider starting or titrating a controller agent. Non-Formulary: ProAir, Proventil, Albuterol syrup, Albuterol tabletsLevalbuterol Tartrate (Xopenex HFA) Coverage Criteria: Xopenex HFA is step therapy to treatment failure or intolerance of AlbuterolSulfate HFA. Limits: None Required Information for Approval: Chart notes with clinical documentation describingintolerance to Albuterol HFA. Other Notes: Use of more than 7 inhalers per 180 day period may indicate uncontrolled asthma.Consider starting or titrating a controller agent.Short Acting AnticholinergicsIpratropium bromide (Atrovent HFA) Coverage Criteria: None Limits: 2 inhalers per 30 days Required Information for Approval: N/A Other Notes: Usage above the quantity limit may indicate uncontrolled disease. Consider adding ortitrating a controller agent.Inhaled CorticosteroidFluticasone Propionate (Flovent HFA/Diskus), Fluticasone Furoate (Arnuity Ellipta), Mometasone Furoate(Asmanex Twisthaler/HFA), Beclomethasone Dipropionate (Qvar), Budesonide (Pulmicort Flexhaler),Flunisolide (Aerospan), Ciclesonide (Alvesco)Fluticasone Propionate (Flovent HFA/Diskus), Beclomethasone Dipropionate (Qvar) Coverage Criteria: None Limits: 1 inhaler/device per 30 days Required Information for Approval: N/A Other Notes: None Non-Formulary: Flunisolide (Aerospan), (Ciclesonide (Alvesco)Fluticasone Furoate (Arnuity Ellipta) Coverage Criteria: Fluticasone Furoate (Arnuity Ellipta) is reserved for patients 12 years and older. Limits: 1 inhaler per 30 days Required Information for Approval: N/A Other Notes: NoneMometasone Furoate (Asmanex Twisthaler), Budesonide (Pulmicort Flexhaler 180 mcg) Coverage Criteria: Mometasone Furoate (Asmanex Twisthaler) 110 mcg and Budesonide (PulmicortFlexhaler) 180 mcg are reserved for patients under the age of 12. Limits: 1 inhaler/device per 30 days Required Information for Approval: N/A Other Notes: Asmanex Twisthaler 220 mcg has no age restriction. Non-Formulary: Asmanex HFA, Pulmicort Flexhaler 90 mcgCoverage Policy – Respiratory Disorders – Asthma & COPDPage 5
Long Acting Beta AgonistSalmeterol Xinafoate (Serevent Diskus), Formoterol Fumarate (Foradil Aerolizer), Indacaterol Maleate(Arcapta Neohaler), Olodaterol Hydrochloride (Striverdi Respimat)Olodaterol HCl (Striverdi Respimat) and Formoterol Fumarate (Foradil Aerolizer) Coverage Criteria: Olodaterol HCl (Striverdi Respimat) and Formoterol Fumarate (ForadilAerolizer) are step therapy to Inhaled Corticosteroid use. Limits: 1 inhaler/package per 30 days. Concurrent use of Inhaled Corticosteroid required. Required Information for Approval: N/A Other Notes: Due to an increased risk of asthma related death, LABAs are not recommended formonotherapy in asthma. Foradil Aerolizer was discontinued by the manufacturer in October 2015.Marketing end date is scheduled for 1/31/17. Non-Formulary: Indacaterol Maleate (Arcapta Neohaler), Salmeterol Xinafoate (Serevent Diskus)Long Acting AnticholinergicTiotropium Bromide (Spiriva, Spiriva Respimat), Aclidinium Bromide (Tudorza Pressair), UmeclidiniumBromide (Incruse Ellipta)For COPDTiotropium Bromide (Spiriva/Spiriva Respimat 2.5mcg), Aclidinium Bromide (Tudorza Pressair) Coverage Criteria: Spiriva, Spiriva Respimat 2.5mcg, and Tudorza Pressair are reserved for patientswith at least GOLD Grade II (moderate airflow limitation) COPD confirmed by PFTs. Limits: Spiriva Respimat 2.5 mcg and Tudorza Pressair: 1 package per 30 days Required Information for Approval: Chart notes with clinical documentation of Moderate (GradeII) COPD diagnosis (i.e., numerical values of PFT results). 2016 GOLD guidelines define Moderate(Grade II) COPD as FEV1/FVC 0.70 and postbronchodilator FEV1 between 50-80% predicted. Other Notes: Long-Acting Anticholinergics should not be used in combination with CombiventRespimat due to the increased risk of anticholinergic side effects. Non-Formulary: Umeclidinium Bromide (Incruse Ellipta)For AsthmaTiotropium Bromide (Spiriva Respimat 1.25mcg) Coverage Criteria: Spiriva Respimat 1.25mcg is step therapy to Montelukast AND one of thefollowing: Symbicort (160 mcg/4.5 mcg), Advair (500 mcg/50 mcg), or Dulera (200 mcg/5 mcg)within the last 30 days. Limits: None Required Information for Approval: Fills of Montelukast and one of the following: Symbicort (160mcg/4.5 mcg), Advair (500 mcg/50 mcg), or Dulera (200 mcg/5 mcg) within the last 30 days. Other Notes: Criteria applies only to Spiriva Respimat 1.25 mcg. Spiriva Respimat and SpirivaHandihaler are restricted for COPD use only.Leukotriene Receptor AntagonistMontelukast Sodium (Singulair), Zafirlukast (Accolate)Montelukast Sodium (Singulair) Coverage Criteria: None Limits: 30 tablets per 30 days Required Information for Approval: N/A Other Notes: None Non-Formulary: Zafirlukast (Accolate)Coverage Policy – Respiratory Disorders – Asthma & COPDPage 6
Xanthine/Phosphodiesterase Enzyme Inhibitor, NonselectiveTheophylline (Theo-24, Elixophyllin, Theochron)Theophylline 80mg/15mL Oral Elixir/Solution; 100 mg, 200 mg, 300 mg, ER capsules (Theo-24); 100mg, 200 mg, 300 mg ER tablets (Theochron, 12-hour); 600 mg ER tablets (24-hour); 450 mg ER tablets(Theochron, 12-hour) Coverage Criteria: None Limits: None Required Information for Approval: N/A Other Notes: Theophylline should be initiated and monitored by an experienced physician, due tothe narrow therapeutic window. Non-Formulary: Theophylline IV Solution, Theo-24 400 mg ER capsulesPDE-4 InhibitorRoflumilast (Daliresp) Coverage Criteria: Daliresp is reserved for patients with GOLD Grade III COPD (post bronchodilatorFEV1/FVC 0.70 and FEV1 of 30-50% predicted) or higher who are compliant with, or intolerant to,use of [1] Long acting anticholinergics (Spiriva) AND [2] ICS (Qvar/Flovent/ArnuityEllipta/Pulmicort) Long acting beta agonists (Serevent/Foradil) or ICS/LABA combination(Advair/Symbicort/Dulera). Limits: None Required Information for Approval: Evidence of compliant use of all other controller medications,in the form of pharmacy fill history. Chart notes detailing a diagnosis of GOLD Grade III COPD,evidenced by Pulmonary Function Testing. Other Notes: NoneMonoclonal AntibodyOmalizumab (Xolair), Mepolizumab (Nucala), Reslizumab (Cinqair)Omalizumab (Xolair) Coverage Criteria: For asthma, Xolair is reserved for poorly controlled moderate-severe allergicasthma patients with baseline serum IgE levels between 30-700 IU/ml, with FEV1 80% predicted,despite being compliant with dose-optimized [1] Inhaled Corticosteroids (ICS) Long-Acting Beta-2Agonist (LABA), [2] Spiriva Respimat, and [3] leukotriene modifier or theophylline. Limits: None Required Information for Approval: Patients must meet all of the following criteria:o Asthma classified as moderate to severe persistent asthmao Pretreatment level of IgE 30IU/ml and 700IU/mlo Positive skin test of in vitro reactivity to at least 1 perennial aeroallergeno Dose optimized inhaled corticosteroids without adequate asthma control (as evidenced byfill history and clinic documentation)o Dose optimized combination inhaled corticosteroid/long-acting beta2-agonist andleukotriene modifier or theophylline. Other Notes: Initial approval is 6 months. Continuing Approval will require updated clinic noteswith documented therapeutic response in the form of improved symptomology. Perennialaeroallergens include: cat or dog dander, house-dust mites, and pollens. Evidence is limited for moldsand cockroaches.2Mepolizumab (Nucala) Coverage Criteria: Nucala is reserved for patients with poorly controlled, severe eosinophilicasthma with baseline serum eosinophil counts of either 150 cells/µL at initiation of treatment or 300 cells/µL in the past 12 months AND 2 or more exacerbations in the past 12 months, despitebeing compliant with dose-optimized [1] Inhaled Corticosteroids (ICS) Long-Acting Beta-2 Agonist(LABA), [2] Spiriva Respimat, and [3] leukotriene modifier or theophylline. Must be prescribed by anallergist. Limits: NoneCoverage Policy – Respiratory Disorders – Asthma & COPDPage 7
Required Information for Approval: Patients must meet all of the following criteria:o Diagnosis of asthmao Eosinophil level of either 150 cells/µL at initiation of treatment or 300 cells/µL in thepast 12 monthso 2 or more exacerbations in the past 12 months, despite being compliant with dose-optimized[1] Inhaled Corticosteroids (ICS) Long-Acting Beta-2 Agonist (LABA), [2] Spiriva Respimat,and [3] leukotriene modifier or theophylline. Other Notes: Initial approval is 6 months. Continuing Approval will require updated clinic noteswith documented therapeutic response in the form of improved symptomology. Non-Formulary: Reslizumab (Cinqair)Short Acting CombinationIpratropium/Albuterol (Combivent Respimat) Coverage Criteria: None Limits: 1 Inhaler per 30 days Required Information for Approval: None Other Notes: Should not be used with Tiotropium (Spiriva).Long Acting CombinationFluticasone/Salmeterol (Advair), Budesonide/Formoterol (Symbicort), Mometasone/Formoterol (Dulera),Fluticasone/Vilanterol (Breo Ellipta), Tiotropium/Otodaterol (Stiolto Respimat), Umeclidinium/ Vilanterol (AnoroEllipta), Glycopyrrolate/ Indacaterol (Utibron Neohaler), Glycopyrrolate/ Formoterol (Bevespi Aerosphere)Fluticasone/Salmeterol (Advair), Budesonide/Formoterol (Symbicort), Mometasone/Formoterol(Dulera) Coverage Criteria: None Limits: 1 Inhaler per 30 days Required Information for Approval: None Other Notes: None Non-Formulary: Fluticasone/Vilanterol (Breo Ellipta)Tiotropium/Otodaterol (Stiolto Respimat) Coverage Criteria: Stiolto Respimat is reserved for patient with at least Grade II (moderate) COPDconfirmed by pulmonary function testing (PFTs). Limits: 1 Inhaler per 30 days Required Information for Approval: PFTs showing post-bronchodilator FEV1/FVC is 0.7 andFEV1 between 50-80% predicted. Other Notes: None Non-Formulary: Umeclidinium/ Vilanterol (Anoro Ellipta), Glycopyrrolate/ Indacaterol (UtibronNeohaler), Glycopyrrolate/ Formoterol (Bevespi Aerosphere)Solution for NebulizationAlbuterol Sulfate, Ipratropium-Albuterol (Duoneb), Ipratropium Bromide, Levalbuterol Hydrochloride,Budesonide, Cromolyn Sodium, Formoterol Fumarate Dihydrate (Perforomist), Arformoterol (Brovana)Albuterol Sulfate, Ipratropium-Albuterol (Duoneb) Coverage Criteria: None Limits: 375mL per 30 days Required Information for Approval: N/A Other Notes: NoneIpratropium Bromide Coverage Criteria: None Limits: None Required Information for Approval: N/A Other Notes: NoneCoverage Policy – Respiratory Disorders – Asthma & COPDPage 8
Levalbuterol Hydrochloride Coverage Criteria: Step Therapy to treatment failure of or intolerance to Albuterol Sulfate Limits: None Required Information for Approval: Chart notes with clinical documentation explainingintolerance to Albuterol. Other Notes: Formoterol Fumarate Dihydrate (Perforomist), Arformoterol (Brovana)Budesonide Coverage Criteria: Restricted to members less than or equal to 4 years of age. Limits: 120 mL per 30 days Required Information for Approval: N/A Other Notes: Members older than 4 should use a mask and spacer to facilitate delivery of ICSproducts. Formulary agents include Qvar, Flovent HFA/Diskus, and Asmanex Twisthaler.Cromolyn Sodium Coverage Criteria: None Limits: None Required Information for Approval: N/A Other Notes: NoneMedical EquipmentPeak Flow Meter, Mask/Spacer, NebulizerPeak Flow Meter, Bubbles the Fisk II Pedi Mask, Nebulizer Coverage Criteria: None Limits: 1 per lifetime Required Information for Approval: N/A Other Notes: Nebulizers will be paid at a maximum of 100 per machine.Optichamber Adult Mask (Large), Optichamber Diamond with Mask, Vortex Holding Chamberwith/without mask Coverage Criteria: None Limits: 2 per year Required Information for Approval: N/A Other Notes: None Non-Formulary: Aerochamber Plus Flow-VU/Plus Z-Stat/Z-stat Plus with mask, Inspirachamberwith mask, Easivent Holding Chamber with maskCoverage Policy – Respiratory Disorders – Asthma & COPDPage 9
Clinical Justification:Figure 1: Global Initiative for Asthma Management and Prevention Strategy 20161*Not for children 12 years. **For children 6–11 years, the preferred Step 3 treatment is medium dose ICS. # Low dose ICS/formoterol isthe reliever medication for patients prescribed low dose budesonide/formoterol or low dose beclometasone/formoterol for maintenanceand reliever therapy. Tiotropium by mist inhaler is an add-on treatment for patients with a history of exacerbations*.Coverage Policy – Respiratory Disorders – Asthma & COPDPage 10
Figure 2: National Asthma Education and Prevention Program Asthma Treatment Guidelines 20122Abbreviations: EIB, exercise-induced bronchospasm† Treatment options are listed in alphabetical order, if more than one.‡ If alternative treatment is used and response is inadequate, discontinue and use preferred treatment before stepping up.§ Theophylline is a less desirable alternative because of the need to monitor serum concentration levels.Based on evidence for dust mites, animal dander, and pollen; evidence is weak or lacking for molds and cockroaches. Evid ence isstrongest for immunotherapy with single allergens. The role of allergy in asthma is greater in children than in adults.†† Clinicians who administer immunotherapy or omalizumab should be prepared to treat anaphylaxis that may occur.‡‡ Zileuton is less desirable because of limited studies as adjunctive therapy and the need to monitor liver function.§§ Before oral corticosteroids are introduced, a trial of high-dose ICS LABA either LTRA, theophylline, or zileuton, may be considered,although this approach has not been studied in clinical trials.Coverage Policy – Respiratory Disorders – Asthma & COPDPage 11
Asthma is a dynamic condition requiring constant assessment in order to provide optimal control ofsymptoms. The HPSJ formulary is designed to make controller agents accessible, as these are the mainstay oftherapy according to NAEPP and GINA guidelines. Controller medications for asthma include inhaledcorticosteroids, long-acting beta-2 agonists, leukotriene antagonists, theophylline, cromolyn, and zileuton.New classes of agents have also entered the market in recent years: long-acting anticholinergics (SpirivaRespimat 1.25 mcg) and monoclonal antibodies (Xolair). Since NAEPP and GINA guidelines list these agentsas add-on therapies for patients with severe, uncontrolled disease, they are reserved for patients who havefailed ICS, LABA, and leukotriene antagonists. Xolair is specifically indicated in patients with allergic asthma,and therefore requires additional lab testing to establish medical necessity.Combination ICS/LABA products such as Advair, Symbicort, and Dulera, are available with quantity limits toensure appropriate use. Short acting-inhalers should only be used on an as-needed basis, and therefore havequantity limits to prevent overuse. Frequent use of short-acting inhalers can be an indicator of poorlycontrolled asthma.Short-acting beta-2 agonists (SABAs) are commercially available as oral syrups or tablets. However, theseformulations are not on HPSJ’s formulary due to NAEPP guideline recommendations, which state inhaledroute is preferred because they cause fewer systemic side effects than oral agents. Additionally, oralextended-release tablets have not been adequately studied as adjunctive therapy with ICS. 2Figure 3: Adapted from Global Initiative for Chronic Obstructive Lung Disease 20163PatientGroupABGOLDGrade1 or 2(mildmoderateairflowlimitation)1 or 2(mildmoderateairflowlimitation)FEV1 80%predictedor50% FEV1 80%predicted 80%predictedor50% FEV1 80%predictedExacerbation0-1 peryear0-1 peryearHospitalizationNoNoCATScore 10 10mMRCGradeRecommendedFirst ChoiceAlternative ChoiceOther PossibleTreatments**0-1SAanticholinergicprn orSA beta2agonist prnLA anticholinergicorLA beta2-agonistorSA beta2-agonistand SAanticholinergicTheophylline 2LAanticholinergicorLA beta2agonistLA anticholinergicand LA beta2agonistSA beta2agonist and/orSAanticholinergicTheophyllineLA anticholinergicand LA beta2agonist3 or 430% FEVSA beta2or(severe1 50%ICS LA beta2agonist and/orLA anticholinergicverypredictedagonist orSAC 2 1 100-1and PDE-4severeorLAanticholinergicInhibitorairflow neLA beta2-agonistand PDE-4InhibitorICS LA beta2agonist and LACarbocysteineanticholinergic orICS LA beta2N3 or 430% FEVagonist and PDE-4acetylcysteine(severe1 50%ICS LA beta2inhibitorverypredictedagonist and/ororD 2 1 10 2SA beta2severeorLALA anticholinergicagonist and/orairflow 30%anticholinergicand LA rLA anticholinergicTheophyllineand PDE-4inhibitor**Medications in this column can be used alone or in combination with other options in the First and Alternative Choice columnsCoverage Policy – Respiratory Disorders – Asthma & COPDPage 12
The mainstay of therapy for COPD is ICS, LABA, and long-acting anticholinergics. Spiriva Handihaler, SpirivaRespimat 2.5 mcg, Tudorza, Stiolto Respimat, and Daliresp have only been approved for COPD. According tothe GOLD Guidelines, the diagnosis and grading of COPD is determined by pulmonary function test results.These agents are reserved for patients with GOLD Grade II COPD or higher. Therefore, HPSJ requirespulmonary function testing to ensure appropriate use based on members’ GOLD Grading. 7.18.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2016. Available from:www.ginasthma.org.National Heart, Lung, and Blood Institute. Expert Panel Report 3: Guidelines for the Diagnosis and Management ofAsthma. 2007. Available from: hgdln.pdf.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, andPrevention of Chronic Obstructive Pulmonary Disease. 2016. Available from: www.goldcopd.org.Global Initiative for Chronic Obstructive Lung Disease. Diagnosis of Diseases of Chronic Airflow Limitation: AsthmaCOPD and Asthma-COPD Overlap Sndrome (ACOS). 2016. Available from: www.goldcopd.org.Chung KF, Wenzel SE, Brozek JL, et al. International ERA/ATS guidelines on definition, evaluation and treatment ofsevere asthma. Eur Respir J. 2014;43 (2): 343-373.Food and Drug Administration. FDA News Release: FDA approves Nucala to treat severe sAnnouncements/ucm471031.htm. Updated November 6, 2015.Accessed September 18, 2016.Nucala [Package Insert]. Phila
mcg/4.5 mcg), Advair (500 mcg/50 mcg), or Dulera (200 mcg/5 mcg) within the last 30 days. Aclidinium Bromide (Tudorza Pressair) 400 mcg/act PA; QL 290.32 Documentation of diagnosis of GOLD Grade II COPD is required for approval. Limit 1 package per 30 days. Umeclidinium Bromide (Incruse Ellipta) 62.5 mcg/act NF 327.30











