Transcription
COREMetadata, citation and similar papers at core.ac.ukProvided by Elsevier - Publisher ConnectorORIGINAL ARTICLESafety and Resource Utilization by Non-small Cell LungCancer HistologyResults from the Randomized Phase III Study of Pemetrexed PlusCisplatin versus Gemcitabine Plus Cisplatin in Chemonaïve Patientswith Advanced Non-small Cell Lung CancerSilvia Novello, MD,* Francisco Luis Pimentel, MD,† Jean-Yves Douillard, MD,‡ Mary O’Brien, MD,§Joachim von Pawel, MD,储 John Eckardt, MD,¶ Astra M. Liepa, PhD,# Lorinda Simms, MSc, PStat,**Carla Visseren-Grul, MD,†† and Luis Paz-Ares, MD‡‡Introduction: A prespecified analysis of the large, randomized,phase III study in advanced non-small cell lung cancer showedsignificant improvement in survival for nonsquamous patients treatedwith pemetrexed/cisplatin versus gemcitabine/cisplatin. Selected grade3/4 toxicities and resource utilization favored pemetrexed in the overallpopulation, but detailed safety results by histology have not beenreported.Methods: Treated patients were included in this analysis of safetyby histology. At each cycle, adverse events were assessed, andconcomitant medications, transfusions, and hospitalizations wererecorded. Measures were summarized by histology and comparedbetween arms with Fisher’s exact test.Results: When analyzed by squamous and nonsquamous histology,safety and resource utilization for each treatment arm paralleledthose of the overall population. Selected toxicities did not vary by*Thoracic Oncology Unit, University of Torino, San Luigi Hospital, Torino,Italy; †Health Science Department, Universidade de Aveiro, Aveiro,Portugal; ‡Department d’ Oncologie Medicale, Centre René Gauducheau, Saint Herblain, France; §Lung Unit, Royal Marsden Hospital,Sutton, United Kingdom; 储Klinik für Pneumologie, Asklepios Fachkliniken,München-Gauting, Germany; ¶Department of Oncology/Hematology, Center for Cancer Care and Research, Saint Louis, Missouri; #Global HealthOutcomes - Oncology, Eli Lilly and Company, Indianapolis, Indiana; **Statistics - Oncology, Eli Lilly Canada, Toronto, Canada; ††Research andDevelopment - Oncology, Eli Lilly and Company, Houten, The Netherlands;and ‡‡Servicio de Oncologia Medica, Hospital Universitario Virgen delRocío, Seville, Spain.Disclosure: The authors declare no conflicts of interest.Address for correspondence: Silvia Novello, MD, Thoracic Oncology Unit,University of Torino, San Luigi Hospital, Regione Gonzole, 10, Orbassano, Torino, Italy 10043. E-mail: silvia.novello@unito.itJohn Eckardt is currently at Dava Oncology, Dallas, Texas.Presented in part at the 12th World Conference on Lung Cancer, Seoul,South Korea, September 2– 6, 2007; 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, Illinois, May 30 to June 3,2008; and 13th World Conference on Lung Cancer, San Francisco,California, July 31 to August 4, 2009.Copyright 2010 by the International Association for the Study of LungCancerISSN: 1556-0864/10/0510-16021602histology. Concomitant medication use and hospitalizations werealso very similar to the patterns observed in the overall population.Conclusions: Although previous efficacy analyses showed a significant pemetrexed treatment advantage for nonsquamous patients,results of this analysis indicate that safety and resource utilization donot vary by histology and are consistent with the overall population.The safety and resource utilization of patients treated with pemetrexed/cisplatin are predictable, reproducible, and consistent withthe established favorable safety profile of pemetrexed, regardless ofhistology.Key Words: Cisplatin, Histology, Non-small cell lung cancer,Pemetrexed, Resource utilization, Safety.(J Thorac Oncol. 2010;5: 1602–1608)The majority of patients with non-small cell lung cancer(NSCLC) present with locally advanced (stage IIIB) ormetastatic (stage IV) disease, for which platinum-based doubletchemotherapy is recommended as a first-line treatment.1–3 Platinum doublets comprise cisplatin or carboplatin combined witha third-generation cytotoxic agent such as pemetrexed, gemcitabine, vinorelbine, irinotecan, or taxane.4 –13 Select patients withnonsquamous NSCLC may also be candidates for a platinumdoublet in combination with bevacizumab.2,3 In patients withNSCLC, platinum-based doublets have demonstrated comparable efficacy with a range of toxicity profiles when evaluatedhead-to-head in phase III studies.8,10 –14 Given the diversity oftoxicities, resource utilization data can be beneficial to characterize the impact of various toxicities that may affect the choiceof first-line treatment.The efficacy and tolerability of pemetrexed have beenwell established in thoracic tumors for several indications. Pemetrexed is approved for use in combination with cisplatin forunresectable mesothelioma,15 as a single agent for second-lineand maintenance treatment of nonsquamous NSCLC,16,17 and incombination with cisplatin for first-line treatment of advancednonsquamous NSCLC.14 The latter indication is based on theJournal of Thoracic Oncology Volume 5, Number 10, October 2010
Journal of Thoracic Oncology Volume 5, Number 10, October 2010results of a large phase III study of pemetrexed/cisplatin versusgemcitabine/cisplatin in chemonaïve patients for first-line treatment of stage IIIB/IV NSCLC,14 which demonstrated noninferior efficacy in the intent-to-treat population and better tolerability for pemetrexed/cisplatin. In addition, resource utilizationfavored the pemetrexed combination.In a prespecified analysis of the same study,14 a significant treatment-by-histology interaction was observed, whichshowed a significant survival advantage for patients withnonsquamous histology treated with pemetrexed/cisplatin.18Squamous patients in the study, however, had shorter survivalon pemetrexed/cisplatin compared with gemcitabine/cisplatin. Analyses of other phase III studies in multiple treatmentindications for NSCLC have also shown a treatment-byhistology interaction for pemetrexed.18,19In the primary report of this study, Scagliotti et al.14indicated that safety within the histologic groups was generally consistent with the overall safety results. This articlefurther explores the safety profile and resource utilization ofpemetrexed-based therapy by histology, analyzing the datafrom the phase III, randomized, noninferiority study comparing pemetrexed/cisplatin and gemcitabine/cisplatin in chemonaïve patients with advanced NSCLC.PATIENTS AND METHODSPatientsThe data for this comprehensive analysis of safety andresource utilization are from the randomized, open-label,global, phase III study comparing pemetrexed/cisplatin withgemcitabine/cisplatin, which was previously published.14 Thestudy included chemonaïve patients with stage IIIB or IVNSCLC, an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate bone marrow reserveand organ function. Exclusion criteria included NationalCancer Institute Common Toxicity Criteria20 (version 2.0)grade ⱖ1 peripheral neuropathy, progressive brain metastases, or uncontrolled third-space fluid retention. The protocolwas conducted according to the Declaration of Helsinki andGood Clinical Practice guidelines and was approved by eachparticipating institution’s ethics review board. All patientssigned written informed consent before treatment.Treatment and Study DesignPatients received either pemetrexed (500 mg/m2 intravenously [IV]) followed by cisplatin (75 mg/m2 IV) on day 1 orgemcitabine 1250 mg/m2 on days 1 and 8 with cisplatin (75mg/m2 IV) on day 1. Both regimens were administered every 3weeks, for a maximum of six cycles. All patients receiveddexamethasone prophylaxis and oral folic acid (350 –1000 g)daily and a vitamin B12 injection (1000 g) every 9 weeks,beginning 1 to 2 weeks before the first dose and continuing until3 weeks after the last dose of study treatment.Supportive care was provided at the investigator’s discretion. Use of 5-HT3 antagonists was encouraged. Routineuse of colony-stimulating factors was not permitted, but itcould be administered according to American Society ofClinical Oncology guidelines.21 Use of erythropoiesis-stimulating agents (ESAs) was permitted, with no specific guide-Safety and Resource Utilization by NSCLC Histologylines or recommendations. Efficacy analyses, including byhistologic groups, have been previously reported.14,18,19,22Safety and Resource Utilization Assessmentsand AnalysesFor safety and resource utilization, patients were assessed at each cycle and at 30 days after poststudy discontinuation. Adverse events (AEs) were assessed using NationalCancer Institute Common Toxicity Criteria version 2.0. Causality was determined by the investigator. The evaluation ofresource utilization included concomitant medications, transfusions, and AE-related hospitalizations. Investigatorreported NSCLC histology was grouped for statistical analysis into four main categories: adenocarcinoma, large cellcarcinoma, squamous cell carcinoma, and other NSCLC/nototherwise specified, consistent with published guidelines.23,24For these analyses, resource utilization and drug-related AEswere grouped and evaluated by squamous and nonsquamous(adenocarcinoma, large cell carcinoma, and other/not otherwise specified) NSCLC histology and compared betweenarms with Fisher’s exact test. Safety analyses were performedfor patients who received at least one dose of study treatment.RESULTSPatient CharacteristicsA total of 1725 patients (pemetrexed/cisplatin 862,gemcitabine/cisplatin 863) were enrolled from July 2004 toDecember 2005. The majority of patients, 1051 (61%), werefrom Europe. Safety analyses were done for 97.3% (839patients) and 96.2% (830 patients) of the patients treated withpemetrexed/cisplatin and gemcitabine/cisplatin, respectively.The baseline patient and disease-related characteristics werewell balanced between the two treatment arms and betweenthe squamous and nonsquamous histologic groups (Table 1).TreatmentThe number of cycles of pemetrexed/cisplatin and gemcitabine/cisplatin delivered were similar between arms andbetween the two histologic groups. On both treatment arms,the median number of cycles was five for all patients and fornonsquamous patients; however, the median was different forsquamous patients: four for patients on the pemetrexed/cisplatin arm and five for patients on the gemcitabine/cisplatin arm.The relative mean dose intensity was similar whencomparing all patients, squamous patients, and nonsquamouspatients, with approximately 1% difference in the mean doseintensity between histologic groups for a given drug (Table 2). The mean dose intensity for gemcitabine was consistently lower than that for pemetrexed (e.g., 85.8% versus94.8% for all patients), as was the mean dose intensity forcisplatin on the gemcitabine arm compared with the meandose intensity for cisplatin on the pemetrexed arm (e.g.,93.5% versus 95.0% for all patients).SafetyThe incidence of selected grade 3/4 toxicities did notvary by histologic group and paralleled results in the overallCopyright 2010 by the International Association for the Study of Lung Cancer1603
Journal of Thoracic Oncology Volume 5, Number 10, October 2010Novello et al.TABLE 1. Pemetrexed Plus Cisplatin versus Gemcitabine Plus Cisplatin: Baseline Patient andDisease Characteristics for Randomized Patients by Histologic e/CisplatinNonsquamous(n ⴝ 618)Squamous(n ⴝ 244)Nonsquamous(n ⴝ 634)Squamous(n ⴝ 9/12/1063.321/8182/7/1128/7231/6980/11/9Median age (yr)Female/male (%)Ever/never smoker/unknown (%)Stage IIIB/IV (%)ECOG PS 0/1 (%)White/Eastern Asian/other (%)aBecause of rounding, percentages may not total 100.ECOG PS, Eastern Cooperative Oncology Group performance status.TABLE 2. Pemetrexed Plus Cisplatin versus GemcitabinePlus Cisplatin: Dose IntensityMean Dose Intensity 186.193.294.194.885.094.4population (Table 3). The incidence of neutropenia, thrombocytopenia, and febrile neutropenia was significantly lowerfor patients treated with pemetrexed/cisplatin in both histologic groups and in the overall population. The incidence ofanemia was significantly lower for nonsquamous patientstreated with pemetrexed/cisplatin compared with nonsquamous patients treated with gemcitabine/cisplatin, which paralleled the overall population. Squamous patients treated withpemetrexed/cisplatin had a slightly lower rate of anemiacompared with those treated with gemcitabine/cisplatin; however, it was not statistically significant. The incidence ofinfections with and without grade 3/4 neutropenia was lowTABLE 3. Pemetrexed Plus Cisplatin versus Gemcitabine Plus Cisplatin: Selecteda Grade 3/4 Toxicities inTreated PatientsOverallGrade 3/4 ToxicityAnemia (%)pNeutropenia (%)pThrombocytopenia (%)pFebrile neutropenia (%)pInfection with grade 3/4 neutropenia (%)pInfection without grade 3/4 neutropenia (%)pFatigue (%)pNausea (%)pVomiting (%)pAnorexia (%)pNonsquamousSquamousPC(n ⴝ 839)GC(n ⴝ 830)PC(n ⴝ 604)GC(n ⴝ 609)PC(n ⴝ 235)GC(n ⴝ 221)5.69.95.010.27.29.0 0.0010.00115.126.714.9 1.0004.95.00.0280.30.30.1437.21.30.6860.717.6 0.0013.30.50.7736.75.10.0340.729.9 0.00110.81.31.0000.615.7 0.0010.0020.725.6 0.001 0.0011.30.4973.81.40.143aSelection of toxicities based on association with resource utilization presented.PC, pemetrexed/cisplatin; GC, gemcitabine/cisplatin.1604Copyright 2010 by the International Association for the Study of Lung Cancer
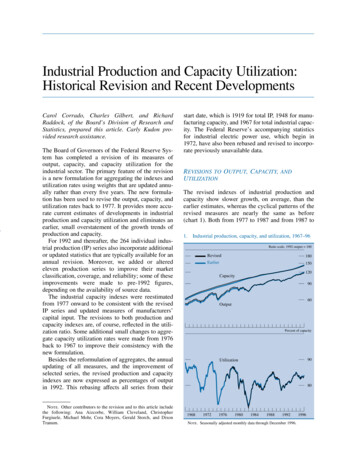
Journal of Thoracic Oncology Volume 5, Number 10, October 2010Safety and Resource Utilization by NSCLC HistologyFIGURE 1. Pemetrexed plus cisplatin versus gemcitabine plus cisplatin: use of antiemetics (A), erythropoiesis-stimulatingagents (B), G-CSF/GM-CSF (C), and antibiotics (D). G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; PC, pemetrexed/cisplatin; GC, gemcitabine/cisplatin.for all patients, regardless of treatment, and was similarbetween treatment and histologic groups.In the overall population, anorexia and nausea weresignificantly higher for pemetrexed/cisplatin than gemcitabine/cisplatin; higher rates were also observed for pemetrexed/cisplatin in both histologic groups (significantly higher fornonsquamous patients treated with pemetrexed/cisplatin compared with gemcitabine/cisplatin). The incidence of vomitingwas similar between treatment arms and histologic groups.Fatigue was numerically worse for pemetrexed/cisplatin;however, the difference was not statistically significant.Investigators categorized each death that occurred during study treatment as because of study disease, possiblybecause of study drug, or because of other causes. Overall,relatively few deaths (7%) occurred during study treatment.Deaths that occurred during treatment that were attributed tostudy-drug toxicity were similar between arms, occurring innine patients (1.0%) on pemetrexed/cisplatin and six patients(0.7%) on gemcitabine/cisplatin. On the pemetrexed/cisplatinarm, six of the nine deaths were in patients with nonsquamous histology, whereas all the deaths on the gemcitabine/cisplatin arm were in patients with nonsquamous histology.Concomitant Therapy and HospitalizationsThe use of concomitant medications is presented inFigure 1. The use of granulocyte colony-stimulating factor/granulocyte macrophage colony-stimulating factors (G-CSF/GM-CSF) and ESAs was significantly lower (p ⱕ 0.012) forthe pemetrexed/cisplatin arm in both histologic groups, withthe exception of the G-CSF/GM-CSF squamous group (p 0.141). The use of antiemetics and antibiotics in the squamous and nonsquamous groups was similar to that in theoverall population, with the only significant difference (p 0.040) being higher use of antiemetics for squamous patientson the pemetrexed/cisplatin arm relative to the gemcitabine/cisplatin arm (Figure 1).The use of transfusions was significantly lower on thepemetrexed/cisplatin arm in the overall population and inboth histologic groups (p ⱕ 0.016; Figure 2). Most of thetransfusions received were red blood cell transfusions.Hospitalizations because of AEs, both regardless ofcausality and drug-related hospitalizations, in the squamousand nonsquamous groups followed the same hospitalizationpattern as in the overall population and were not significantlydifferent between treatment arms (Figure 3). Hospitalizations,regardless of causality, ranged from 31.1 to 37.1% for allgroups, with slightly fewer hospitalizations for the pemetrexed/cisplatin arm. Drug-related hospitalizations represented less than half of the total hospitalizations and wereslightly lower for the pemetrexed/cisplatin arm in the overallCopyright 2010 by the International Association for the Study of Lung Cancer1605
Journal of Thoracic Oncology Volume 5, Number 10, October 2010Novello et al.FIGURE 2. Pemetrexed plus cisplatin versus gemcitabineplus cisplatin: transfusions. PC, pemetrexed/cisplatin; GC,gemcitabine/cisplatin.and squamous groups, but they were numerically higher (14.4versus 13.0%) for the pemetrexed/cisplatin arm in thenonsquamous group.DISCUSSIONIn a prespecified analysis of the large, randomized,noninferiority study comparing pemetrexed/cisplatin withgemcitabine/cisplatin in the first-line treatment of advancedNSCLC, nonsquamous patients treated with pemetrexed/cisplatin showed a significant improvement in survival compared with gemcitabine/cisplatin. Squamous patients hadshorter survival on pemetrexed/cisplatin compared with gemcitabine/cisplatin.14,18 An analysis of treatment-by-histologyinteraction in the study indicated a differential treatment effectfor pemetrexed/cisplatin according to histology.18 Analyses ofboth second-line and maintenance therapy studies have alsodemonstrated a favorable pemetrexed treatment effect in patientswith advanced nonsquamous NSCLC.17–19,22 Although a definitive explanation for this pemetrexed treatment effect remainsunknown, many discussions suggest that it is based on thebiology of NSCLC tumors.18,25–30The differential effect for pemetrexed that was shownpreviously for efficacy parameters, however, was not observedfor safety and resource utilization measures when analyzedaccording to NSCLC histology. In both this study and the large,phase III pemetrexed maintenance study in NSCLC, resourceutilization and AEs in the histologic groups were generallyconsistent with those in the overall population.14,17,31In the overall population of the pemetrexed/cisplatinversus gemcitabine/cisplatin study, the pemetrexed/cisplatinarm demonstrated a more favorable safety profile and lessresource utilization compared with the gemcitabine/cisplatinarm.14 There were significantly lower incidences of drugrelated grade 3/4 hematologic toxicities and febrile neutropenia, even though the patients on both arms received a similarnumber of treatment cycles. In addition, patients treated withpemetrexed/cisplatin required significantly fewer transfusionsand hematologic supportive care interventions (i.e., ESAs andG-CSF/GM-CSF) than did those on the gemcitabine/cisplatinarm. The lack of difference in the use of antiemetics suggeststhat prophylaxis was used consistently between arms, whichreflects standard practice for cisplatin administration. Antibiotic use and non– drug-related hospitalizations also did notdiffer between arms, perhaps because of associated comorbidities and disease-related complications (such as pneumonia) in this population. Although a higher rate of hospitalization in the gemcitabine/cisplatin arm might be expected, thehigher rates of transfusions and growth factor use on thegemcitabine/cisplatin arm may have decreased the need forhospitalizations because of hematologic toxicity.In this analysis of safety and resource utilization according to histology, the same favorable toxicity profile forpemetrexed/cisplatin emerged as that seen in the overallpopulation, accompanied by a similar reduction in resourceutilization in both histologic groups. When analyzed according to squamous and nonsquamous histology, safety andresource utilization measures paralleled those of the overallpopulation. In the histologic groups, the baseline patient anddisease characteristics, number of treatment cycles delivered,dose intensity, incidence of grade 3/4 toxicities, concomitanttherapy administration, and hospitalizations were very similar tothe patterns observed in the overall population. Although numerically consistent, the lack of statistical significance in thesquamous group may be attributed to its smaller sample size.Given that this was an international trial conducted in26 countries, patterns of resource utilization would be expected to vary by country. Additional analyses of resourceFIGURE 3. Pemetrexed plus cisplatin versus gemcitabine plus cisplatin: hospitalizations because of adverse events, regardlessof causality (A) and drug-related (B). PC, pemetrexed/cisplatin; GC, gemcitabine/cisplatin.1606Copyright 2010 by the International Association for the Study of Lung Cancer
Journal of Thoracic Oncology Volume 5, Number 10, October 2010utilization and safety in this study by geographic region,however, demonstrated a consistent pattern that favored pemetrexed/cisplatin.32,33One major limitation to these analyses should be noted.This phase III trial was designed with overall survival as itsprimary endpoint and was powered accordingly to demonstrate noninferiority. As such, the study was not designed orpowered to detect differences in safety or resource utilizationin the overall population or in the histologic groups. Thus, pvalues are presented here to aid the reader in determining thestrength of the findings presented and should be consideredwith all available information regarding pemetrexed/cisplatinand gemcitabine/cisplatin.The results of these analyses suggest that the observedsafety and resource utilization reflect a host-based phenomenon; that is, they are the result of individual patient toleranceof therapy rather than an expression of tumor biology. It maybe beneficial to explore these observations further by analyzing more phase III randomized studies in advanced NSCLCaccording to histology. However, the consistency of theresults presented here, in addition to those for the large phaseIII maintenance study of pemetrexed,18,19,22 is compelling.There is a significant survival advantage for patientswith nonsquamous histology treated with pemetrexed, for whichtumor biology-based hypotheses have been suggested.18,25–30However, our analyses indicate that the safety and resourceutilization for patients with advanced NSCLC do not varyaccording to histology. These results demonstrate that the safetyprofile of pemetrexed/cisplatin therapy is similar regardless ofhistologic group. In general, the safety and resource utilizationof patients treated with pemetrexed/cisplatin are predictable,reproducible, and consistent with the established safety profile ofpemetrexed. These results reflect the favorable safety profile ofpemetrexed/cisplatin, with low use of transfusions and concomitant medications, regardless of histologic group.ACKNOWLEDGMENTSSupported by Eli Lilly and Company.The authors thank all the patients, investigators, andinstitutions that were involved in this study. They also thankPatti Moore and Noelle Gasco of Eli Lilly and Company forassistance with manuscript preparation.REFERENCES1. Bunn PA Jr, Kelly K. New combinations in the treatment of lung cancer.A time for optimism. Chest 2000;117(4 Suppl 1):138S–143S.2. NCCN Clinical Practice Guidelines in Oncology : Non-small cell lungcancer. National Comprehensive Cancer Network, 2009. Available at:http://www.nccn.org/professionals/physician gls/f guidelines.asp?button I Agree#site. Accessed June 19, 2009.3. D’Addario G, Felip E; ESMO Guidelines Working Group. Non-smallcell lung cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20(Suppl 4):68 –70.4. Manegold C, Gatzemeier U, von Pawel J, et al. Front-line treatment ofadvanced non-small-cell lung cancer with MTA (LY231514, pemetrexed disodium, ALIMTA) and cisplatin: a multicenter phase II trial.Ann Oncol 2000;11:435– 440.5. Shepherd FA, Dancey J, Arnold A, et al. Phase II study of pemetrexeddisodium, a multitargeted antifolate, and cisplatin as first-line therapy inpatients with advanced nonsmall cell lung carcinoma: a study of theNational Cancer Institute of Canada Clinical Trials Group. Cancer2001;92:595– 600.Safety and Resource Utilization by NSCLC Histology6. Scagliotti GV, Kortsik C, Dark GG, et al. Pemetrexed combined withoxaliplatin or carboplatin as first-line treatment in advanced non-smallcell lung cancer: a multicenter, randomized, phase II trial. Clin CancerRes 2005;11(2 Pt 1):690 – 696.7. Zinner RG, Fossella FV, Gladish GW, et al. Phase II study of pemetrexed in combination with carboplatin in the first-line treatment ofadvanced nonsmall cell lung cancer. Cancer 2005;104:2449 –2456.8. Scagliotti GV, De Marinis F, Rinaldi M, et al.; Italian Lung CancerProject. Phase III randomized trial comparing three platinum-baseddoublets in advanced non-small-cell lung cancer. J Clin Oncol 2002;20:4285– 4291.9. Le Chevalier T, Scagliotti G, Natale R, et al. Efficacy of gemcitabineplus platinum chemotherapy compared with other platinum containingregimens in advanced non-small-cell lung cancer: a meta-analysis ofsurvival outcomes. Lung Cancer 2005;47:69 – 80.10. Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational,phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX326 study group. J Clin Oncol 2003;21:3016 –3024.11. Kelly K, Crowley J, Bunn PA Jr, et al. Randomized phase III trial ofpaclitaxel plus carboplatin versus vinorelbine plus cisplatin in thetreatment of patients with advanced non–small-cell lung cancer: aSouthwest Oncology Group trial. J Clin Oncol 2001;19:3210 –3218.12. Schiller JH, Harrington D, Belani CP, et al; Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advancednon-small-cell lung cancer. N Engl J Med 2002;346:92–98.13. Ohe Y, Ohashi Y, Kubota K, et al. Randomized phase III study ofcisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plusgemctabine, and cisplatin plus vinorelbine for advanced non-small-celllung cancer: four-arm cooperative study in Japan. Ann Oncol 2007;18:317–323.14. Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparingcisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer.J Clin Oncol 2008;26:3543–3551.15. Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study ofpemetrexed in combination with cisplatin versus cisplatin alone inpatients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636 –2644.16. Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial ofpemetrexed versus docetaxel in patients with non-small-cell lung cancerpreviously treated with chemotherapy. J Clin Oncol 2004;22:1589 –1597.17. Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexedplus best supportive care versus placebo plus best supportive care fornon-small-cell lung cancer: a randomised, double-blind, phase 3 study.Lancet 2009;374:1432–1440.18. Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy ofpemetrexed according to NSCLC histology: a review of two phase IIIstudies. Oncologist 2009;14:253–263.19. Scagliotti GV, Brodowicz T, Shepherd FA, et al. Pemetrexed is moreeffective in patients with nonsquamous non-small cell lung cancer(NSCLC) histology: an analysis of three large, randomized, phase IIItrials. Presented at the 13th World Conference on Lung Cancer, SanFrancisco, CA, July 31–August 4, 2009 (abstract B2.6).20. National Cancer Institute (NCI). Cancer Therapy Evaluation Program(CTEP). Common Toxicity Criteria (CTC) version 2.0, 1999. Availableat: nic applications/ctc.htm#ctc 20. Accessed May 8, 2008.21. Ozer H, Armitage JO, Bennett CL, et al; American Society of ClinicalOncology. 2000 update of recommendations for the use of hematopoieticcolony-stimulating factors: evidence-based, clinical practice guidelines.American Society of Clinical Oncology Growth Factors Expert Panel.J Clin Oncol 2000;18:3558 –3585.22. Belani CP, Brodowicz T, Ciuleanu T, et al. Maintenance pemetrexed(Pem) plus best supportive care (BSC) versus placebo (Plac) plus BSC:a randomized phase III study in advanced non-small cell lung cancer(NSCLC). J Clin Oncol 2009;27(Suppl 18S):abstract CRA8000.23. Travis WD, Colby TV, Corrin B, et al. Histological Typing of Lung andPleural Tumours (WHO. World Health Organization. International Histological Classification of Tumours), 3rd Ed. Berlin: Springer-Verlag,1999.Copyright 2010 by the International Association for the Study of Lung Cancer1607
Novello et al.Journal of Thoracic Oncology Volume 5, Number 10, October 201024. Non-Small Cell Lung Cancer Treatment (PDQ威). Cellular Classificationof Non-Small Cell Lung Cancer. National Cancer Institute (NCI) website. Available at: on-small-cell-lung/HealthProfessional/page3. Accessed September 20,2009.25. Scagliotti G, Kaiser C, Biesma B, et al. Correlations of biomarkerexpression and clinical outcome in a large phase III trial of pemetrexedplus cisplatin or gemcitabine plus cisplatin in chemonaive patients withlocally advanced or metastatic non-small cell lung cancer (N
histologic groups, have been previously reported.14,18,19,22 Safety and Resource Utilization Assessments and Analyses For safety and resource utilization, patients were as-sessed at each cycle and at 30 days after poststudy discon-tinuation. Adverse events (AEs) were assessed using National Cancer Institute Common Toxicity Criteria version 2.0 .