Transcription
Experiment 6Titration curve of amino acidMona AL-Harbi
Introduction:General rules for amino acid ionization Alpha carboxylic acids ionize at acidic pH and have pKa less than 6;So in titrating a fully protonated amino acid, alpha carboxylic acidslose the proton first. Alpha amino groups ionize at basic pH and have pKa greater than 8;So after acids lose their protons, amino groups lose their proton. Most of the 20 amino acids are similar to alanine (has two pKa values)in their ionization properties because their side chains do not ionize atbiological pH. However, there are 5 exceptions worth noting (the amino acids withpolar charged side chains) Glu, Asp, Lys, Arg, His(basic and acidic amino acids) Each has 3 ionizible groups and thus, 3 pKs.
Titration of amino acid When an amino acid is dissolved in water it existspredominantly in the isoelectric form. Upon titration with acid, it acts as a base, and upontitration with base, it acts as an acid( a compoundthat can act as either an acid or a base is known asan amphoteric compound).
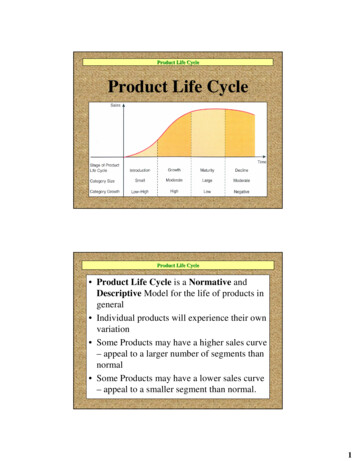
Titration curve: is produced by monitoring the PH of a givenvolume of a sample solution after successive addition ofacid or base.Amino acids are example of weak acid which contain morethan one dissociate group.Example :(1)alanine contain COOH (PKa1 2.34)and NH3 (PKa2 9.69)groups (it has one PI value 6.010)[diprotenation]The COOH will dissociate first then NH3 dissociate later .(Because PKa1 PKa2)(2)Arginine contain COOH (PKa1 2.34) , NH3 (PKa2 9.69) groupsand basic group(pKa3 12.5) (it has one pI value 11)[triprotenation]
(1)Titration curve of alanine (or glycine) [diprotenation]9X6X5X2X1X7X8X3X 4X[1] alanine in starting point is full protenation [NH3 -CH-CH3-COOH][2] COOH will dissociate first ,[NH3 -CH-CH3-COOH] [NH3 -CH-CH3-COO-]PH PKa1[3] [NH3 -CH-CH3-COOH] [NH3 -CH-CH3-COO-] , PH PKa1,We already define Pka in the last experiment , in this point the component ofalanine act as buffer
9X6X7X8X5X2X1X3X 4X[4] [NH3 -CH-CH3-COOH] [NH3 -CH-CH3-COO-] , PH PKa1[5] The COOH full dissociate to COO- , [NH3 -CH-CH3-COO-] .At this point the conc. Of negative charge conc. Of positive charge .the amino acidpresent as Zwetter ion (neutral form) .PI (isoelectric point) : PH value at which the net charge of amino acid equal to zero.PI (PKa1 PKa2) /2 (2.32 9.96)/2 6.01
9X6X7X8X5X2X1X3X 4X[6] the NH3 start dissociate ,[NH3 -CH-CH3-COO-] [NH2-CH-CH3-COO-]PH PKa2[7] [NH3 -CH-CH3-COO-] [NH2-CH-CH3-COO-] . PH PKa2 , the componetntof alanine act as buffer.
9X6X7X8X5X2X1X3X 4X[8] [NH3 -CH-CH3-COO-] [NH2-CH-CH3-COO-] , PH PKa2[9] the NH3 group will dissociate and at the same time the alanine fulldissociate in end point , [NH2-CH-CH3-COO]POH (Pkb P[A-])/2PKb PKw – PKa2
Note in calculation method:The PH calculated by different way :[1] at starting pointPH (Pka P[HA])/2[2] At any point within the curve (befor or in or after middletitration)PH Pka log([A-]/[HA])[3] At end pointPOH (PKb P[A-])/2PH PKw – POH
Objective:1-To study titration curve of amino acids , determine Pka ofionizable groups , amino acid’s PI2-reinforce the understanding of buffer.Method:(A) You are provided with 10 ml of a 0.1M alanine solution,titrate it with 0.1M NaOH adding the base drop wise mixing, andrecording the pH after each 0.5 ml NaOH added untilyou reach a pH 13.ml of 0.1 NaOH00.511.5PH
Method:(B) You are provided with 10 ml of a 0.1M alanine solution,titrate it with 0.1 M HCL adding the aciddrop wise mixing, andrecording the pH after each 0.5 ml HCL added untilyou reach a pH 1.30.1 M HCL00.511.5PH
Results:[1] record the titration table and Plot a Curve of pH versusml of OH- added.[2]Calculate the pH of the alanine solution after the additionof 0 ml, 5ml, of 0.2M NaOH. And calculate PH after additionof 0.5 ml , 2 ml of HCL[3] determine the pKa of ionizable groups of amino acids[4]Compare your calculated pH values with those obtainedfrom Curve.[5] determine the PI value from your result .
(2) Titration curve of arginine [triprotenation]The same alanine (except there are no pI. value)
(2) Titration curve of arginine [triprotenation]X2 X3X1*PI 11.0 the end point for second group dissociate.(NH3 )(1) [R ] [R] (pK2 pH pKa3)(2) [R ] [R]( pKa2 pH pKa3)(3) [R ] [R] ( pKa pH pKa3)
Objective:1-To study titration curve of amino acids , determine Pka ofionizable groups , amino acid’s PI2-reinforce the understanding of buffer.Method:(A) You are provided with 10 ml of a 0.1M arginine solution,titrate it with 0.1M NaOH adding the base drop wise mixing, andrecording the pH after each 0.5 ml NaOH added untilyou reach a pH 13.ml of 0.1 NaOH00.511.5PH
Method:(B) You are provided with 10 ml of a 0.1M arginine solution,titrate it with 0.1 M HCL adding the acid drop wise mixing, andrecording the pH after each 0.5 ml HCL added untilyou reach a pH 1.30.1M HCL00.511.5PH
Results:[1] record the titration table and Plot a Curve of pH versusml of OH- added.[2]Calculate the pH of the alanine solution after the additionof 0 ml, 5ml, of 0.2M NaOH. And calculate PH after additionof 0.5 ml , 2 ml of HCL[3] determine the pKa of ionizable groups of amino acids[4]Compare your calculated pH values with those obtainedfrom Curve.[5] determine the PI value from your result .
Titration curve: is produced by monitoring the PH of a given volume of a sample solution after successive addition of acid or base. Amino acids are example of weak acid which contain more than one dissociate group. Example : (1)alanine contain COOH (PKa1 2.34)and NH3 (PKa2 9.69) groups (it has one PI value 6.010)[diprotenation]