Transcription
Food Sci Biotechnol (2020) 00750-6Supercooling preservation technology in food and biologicalsamples: a review focused on electric and magnetic fieldapplicationsTaiyoung Kang1 Youngsang You2 Soojin Jun2Received: 3 February 2020 / Revised: 27 February 2020 / Accepted: 10 March 2020 / Published online: 28 March 2020Ó The Author(s) 2020, corrected publication 2020Abstract Freezing has been widely recognized as the mostcommon process for long-term preservation of perishablefoods; however, unavoidable damages associated with icecrystal formation lead to unacceptable quality losses duringstorage. As an alternative, supercooling preservation has agreat potential to extend the shelf-life and maintain qualityattributes of fresh foods without freezing damage. Investigations for the application of external electric field (EF)and magnetic field (MF) have theorized that EF and MFappear to be able to control ice nucleation by interactingwith water molecules in foods and biomaterials; however,many questions remain open in terms of their roles andinfluences on ice nucleation with little consensus in theliterature and a lack of clear understanding of the underlying mechanisms. This review is focused on understanding of ice nucleation processes and introducing theapplications of EF and MF for preservation of food andbiological materials.Keywords Food preservation Supercooling Freezing Electric and magnetic field& Soojin Junsoojin@hawaii.eduTaiyoung Kangtaiyoung@hawaii.eduYoungsang Youyyou7@hawaii.edu1Department of Molecular Biosciences and Bioengineering,University of Hawaii at Manoa, Honolulu, Hawaii 96822,USA2Department of Human Nutrition, Food and Animal Sciences,University of Hawaii at Manoa, Honolulu, Hawaii 96822,USAList of symbolsAAmpereACAlternating currentAEFAlternating electric fieldBMagnetic flux densityC0Concentration of nucleation sitesCASCell alive systemCNTClassical nucleation theoryDElectric flux densityDElectric displacementDCDirect currentEElectric field intensityEFElectric fieldEMFElectromagnetic fieldf*Frequency of monomer attachment to nucleusDGnGibbs free energy of a new phaseDG nCritical Gibbs free energy of a new phaseSurface free energy of the nucleiDGSDGVVolumetric free energy of the nucleiHMagnetic field intensityDHfEnthalpy of fusionHzHertzJCurrent densityJNucleation rateJeExternally generated current densitykBBoltzmann constantMFMagnetic fieldNNumber of ice nuclei per unit volumeN*Concentration of nucleiOMFOscillating magnetic fieldPPermanent polarizationrRadiusr Critical radiusSEntropy123
304SEFSMFTTDTTmUVVZee0hll0lrqvrrðS/mÞsvT. Kang et al.Static electric fieldStatic magnetic fieldTeslaTemperatureDegree of supercoolingMelting pointInternal energyVoltageVolumeZeldovich factorPermittivity of the mediumPermittivity of the free spaceWetting anglePermeabilityPermeability of the free spaceRelative permeabilityElectric charge densitySurface energy of the particle per unit volumeElectrical conductivityNucleation induction periodMagnetic susceptibilityIntroductionThe majority of foods are susceptible to spoilage due tomainly the growth of pathogenic bacteria and the enzymatic activities during storage. It is estimated thatapproximately 25–30% of perishable food is wastedbecause of the food spoilage and most of this wastagecould be saved if stored properly (Stonehouse and Evans,2015). Numerous intrinsic and extrinsic factors such asmoisture content, pH, the presence of enzymes, oxygenconcentration, and exposure to light are associated with therate of food spoilage, but it is well agreed on that temperature management is the most effective way to retardbacterial growth and slow chemical reactions (Atlas, 2006;Jakobsen and Bertelsen, 2000; Pérez-Rodrı́guez et al.,2018). Refrigeration and freezing (i.e. cold storage) are theoldest and most widely used methods of preservation.Refrigeration is the process in which heat is removed fromfoods that present at a higher temperature than its surrounding environment. In general, commercial and household refrigerators are operated at below 7 C or a slightlylower temperature above the freezing point of foods.Refrigeration effectively delays spoilage of perishablefoods by decreasing the chemical and biological processes;however, it will only extend the shelf-life for a few days orweeks at the most depending upon the type of product. Bylowering the temperature, water within a food material isinevitably converted to a solid state. Freezing is recognizedas the most popular method currently available for preserving foods and biological materials for an extended123period. Nevertheless, the formation of ice crystals irreversibly affects the microstructure of the frozen foodsbecause the specific volume of ice is substantially greaterthan that of water, thereby ice crystals compress the foodmatrix and cause the undesirable release of exudate afterthawing (Evans, 2009). Furthermore, frozen storage leadsto an increase in the concentration of solutes in theunfrozen phase, which is the main reason responsible forthe quality deterioration of frozen foods such as proteindenaturation, lipid oxidation, and degradation of color,pigment, and flavor (Leygonie et al., 2012; Zaritzky, 2006).Thus, a new technique based on subzero nonfreezingpreservation has been of interest to researchers. Onetechnique that addresses the problems associated withfreezing is known as supercooling.Supercooling is defined as the process of lowering thetemperature of a food material below its equilibriumfreezing point without the formation of ice crystals(Stonehouse and Evans, 2015). There has been continuousinterest in applications of supercooling technology for foodpreservation since it promises an extended shelf-life whileavoiding ice crystal formation and maintaining fresh textural integrity (Shafel, 2015; Stonehouse and Evans, 2015).Previous research has shown the clear benefits of supercooling preservation over the conventional cold storagemethods; however, unfortunately, the supercooled state ishighly unstable and ice nucleation is induced by astochastic process, leading to the difficulty in achieving thetechnical stability and reproducible results (Deora et al.,2013; James et al., 2009). Moreover, since the supercooledstate within foods has been mostly achieved through stricttemperature control, it is extremely challenging to maintainthe supercooled state for a period sufficiently long whenthe products are subjected to external influences such asphysical vibration and temperature fluctuations (Fukumaet al., 2012; Wilson et al., 2003).External electric field (EF) and magnetic field (MF)appear to influence the onset of ice crystal formation duringsupercooling since water consists of dipole molecules andis also a diamagnetic substance (Woo and Mujumdar,2010). Water molecules naturally present in foods tend torealign and re-orientate by the applications of EF and MFeither in tandem with or separately. This behavior indicatesthat EF and MF are potentially able to inhibit ice nucleation and may lead to a substantial change in the supercooling behavior of food products. However, the effects ofEF and MF on the nucleation process remain a highlycontroversial issue because the underlying mechanisms arenot fully elucidated and investigations into wide ranges ofintensities, frequencies, exposure times, etc. are unexplored(Dalvi-Isfahan et al., 2017b; Otero et al., 2016). Moreover,the process of ice nucleation is perceived as an enigmaticphenomenon due to the spontaneous and stochastic nature
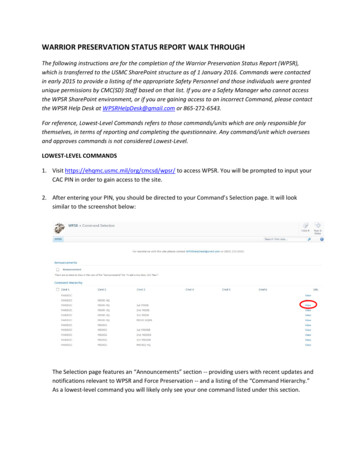
Electric and magnetic fields for supercooled storage305(Hartmann et al., 2013). Therefore, further and deeperinvestigations are needed to draw meaningful conclusionsabout the effects of EF and MF on the control of icenucleation and the extension of supercooling. In thisreview, the concepts of ice nucleation and its significanceon the extension of supercooling are addressed. Additionally, the current literature on the applications of EF and MFfor controlling the nucleation processes are also reviewed.Ice nucleation and supercooling in biologicalsystemsIce nucleation: the first step toward freezingNucleation is the process of the creation of a new thermodynamic phase from an existing phase with high freeenergy to an ordered structure with low free energy. Infreezing, ice nucleation is the key step in determining theoverall freezing characteristics and the quality of finalproducts (Petzold and Aguilera, 2009). There are abundantfundamental and theoretical approaches have been proposed such as phenomenological, kinetic, and microscopicanalyses to properly describe and quantify the nucleationevents; however, it still remains a difficult task because icenucleation occurs at the molecular level with very shorttime scales (Karthika et al., 2016). Although limitations ofclassical nucleation theory (CNT) are inherent, it is considered as the most common theoretical framework andoffers a basic platform to predict a temperature-dependentnucleation rate via thermodynamic and kinetic components. In most cases, ice nucleation in a supercooled liquidoccurs by homogeneous and heterogeneous (Kiani andSun, 2011). Homogeneous nucleation takes place inhomogenous particle-free supercooled liquids when thermal fluctuations in the molecular arrangement can lead tothe spontaneous formation of a stable structure that servesas the critical nucleus without the involvement of foreignsubstances. Homogeneous nucleation rarely occurs in mostsystems because it requires very large supercoolingdegrees; however, it is basically taken into consideration intheoretical approaches due to the complexities of nucleation (Karthika et al., 2016). The Gibbs free energy of aspherical crystallite (new phase, DGn ) in mother phase(supercooled liquid) can be described by the sum of thesurface free energy (DGS Þ and the volumetric free energy(DGV Þ:DGn ¼ DGS þ DGVð1ÞThe change in the free energy of a spherical nucleus ofradius r during homogeneous nucleation (Fig. 1A) is givenby:4DGn ¼ 4pr 2 r pr 3 DGV3ð2Þwhere r is the surface energy of the particle per unit volume. The first and second term of Eq. 2 presents theincrease in energy required to create a new surface and thebulk free energy, respectively (Ickes et al., 2015; Kiani andSun, 2011). Equation 2 indicates that the change in theGibbs free energy depends upon the radius of the nucleus.For the nucleus of smaller r, the first term dominates,leading to the increase in the free energy; while the secondterm governs when r is larger, which leads to the overalldecrease in free energy (Karthika et al., 2016). The criticalradius (r ) is the radius at which the Gibbs free energycurve reaches at the maximum value (Fig. 1B, DG Þ and itcan be obtained by differentiating Eq. 2 with respect to r:r ¼ 0 ¼ 8prr 4pr 2 DGV ¼ 2rDGVð3ÞThen, the critical free energy (DG n ) in which the energybarrier to overcome is written by:DG n ¼16 pr33 DG2Vð4ÞSince water clusters smaller than a critical size areunstable, they spontaneously appear and disappear throughthermal fluctuations. However, once a cluster reaches acritical size (ice embryo), the additional inclusion of watermolecules gradually lowers the free energy and the iceembryo continues to grow, become thermodynamicallyfavored, and towards crystallization (Moore and Molinero,2011). Supercooling acts as a driving force to overcome thefree energy and the magnitude of the force is proportionalto the degree of supercooling (Kiani et al., 2011). Thedifference between the Gibbs free energy of liquid andsolid is proportional to the supercooling below the meltingpoint, then DGV can be rewritten as:DGV ¼DHf DTTmð5Þwhere DHf is the enthalpy of fusion, Tm is the meltingpoint, DT is the degree of supercooling (Tm TÞ. Thus,Eq. 4 can be expressed by:!16 pr3 Tm21 DGn ¼ð6Þ3DHf2 DT 2Equation 6 implies that the high degree of supercoolingdecreases both the critical radius size and the free energy,resulting in an increasing rate of nucleation. The number ofice nuclei (N) per unit volume of a supercooled liquid isexpressed by an Arrhenius type relationship (Ickes et al.,2015; Kiani and Sun, 2011):123
306T. Kang et al.Fig. 1 (A) Homogeneous nucleation. (B) Changes in the free energyof a spherical nucleus of radius r during homogeneous nucleationbased on the CNT (Karthika et al., 2016). (C) Heterogeneousnucleation on a surface. (D) Comparative representation of the Gibbs 3N m DGn¼ N1 expkB T ð7Þwhere N1 is the volume-based number density of watermolecules in the liquid phase, kB is the Boltzmann constant. In the CNT, the nucleation rate (J) is defined as theformation rate of the critical ice nucleus in a unit volume ofwater over time. It is given by combining the thermodynamic and the kinetic terms at the steady-state: 3 1 DG n J m s¼ Zf C0 exp ð8ÞkB Twhere Z is the Zeldovich factor, f is the frequency ofmonomer attachment to the nucleus, C0 is the concentration of nucleation sites (Kiani and Sun, 2011). The kineticpart in Eq. 8 (Zf C0 ) is related to the flux of water molecules available to transfer into the critical ice nucleus and itcan be stated as the diffusive flux, the nucleus surfacecharacteristics, and the kinetic prefactor (Ickes et al., 2015;123free energy for homogeneous (DG hom ) and heterogeneous (DG het )(Çelikbilek et al., 2012)Li et al., 2011). In biological systems, ice nucleation in asupercooled liquid is always heterogeneous (Wilson et al.,2003). In heterogeneous situation, ice nucleation is catalyzed by structural inhomogeneities such as containersurfaces, dislocations, and any solid or liquid impurities incontact with water. These foreign phases significantlyreduce the surface free energy of the nuclei and consequently lower the energy barrier. Figure 1(C) shows aschematic of heterogeneous nucleation on surface materialand rIL , rSL , rSI denotes the interfacial energy of liquidsurface, solid–liquid, and solid-surface
foods; however, unavoidable damages associated with ice crystal formation lead to unacceptable quality losses during storage. As an alternative, supercooling preservation has a great potential to extend the shelf-life and maintain quality attributes of fresh foods without freezing damage. Inves-tigations for the application of external electric field (EF) and magnetic field (MF) have .