Transcription
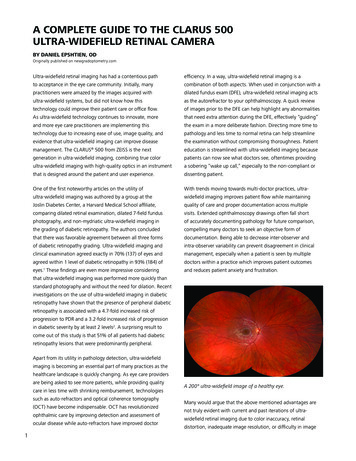
A COMPLETE GUIDE TO THE CLARUS 500ULTRA-WIDEFIELD RETINAL CAMERABY DANIEL EPSHTIEN, ODOriginally published on newgradoptometry.comUltra-widefield retinal imaging has had a contentious pathefficiency. In a way, ultra-widefield retinal imaging is ato acceptance in the eye care community. Initially, manycombination of both aspects. When used in conjunction with apractitioners were amazed by the images acquired withdilated fundus exam (DFE), ultra-widefield retinal imaging actsultra-widefield systems, but did not know how thisas the autorefractor to your ophthalmoscopy. A quick reviewtechnology could improve their patient care or office flow.of images prior to the DFE can help highlight any abnormalitiesAs ultra-widefield technology continues to innovate, morethat need extra attention during the DFE, effectively “guiding”and more eye care practitioners are implementing thisthe exam in a more deliberate fashion. Directing more time totechnology due to increasing ease of use, image quality, andpathology and less time to normal retina can help streamlineevidence that ultra-widefield imaging can improve diseasethe examination without compromising thoroughness. Patientmanagement. The CLARUS 500 from ZEISS is the nexteducation is streamlined with ultra-widefield imaging becausegeneration in ultra-widefield imaging, combining true colorpatients can now see what doctors see, oftentimes providingultra-widefield imaging with high-quality optics in an instrumenta sobering “wake up call,” especially to the non-compliant orthat is designed around the patient and user experience.dissenting patient.One of the first noteworthy articles on the utility ofWith trends moving towards multi-doctor practices, ultra-ultra-widefield imaging was authored by a group at thewidefield imaging improves patient flow while maintainingJoslin Diabetes Center, a Harvard Medical School affiliate,quality of care and proper documentation across multiplecomparing dilated retinal examination, dilated 7-field fundusvisits. Extended ophthalmoscopy drawings often fall shortphotography, and non-mydriatic ultra-widefield imaging inof accurately documenting pathology for future comparison,the grading of diabetic retinopathy. The authors concludedcompelling many doctors to seek an objective form ofthat there was favorable agreement between all three formsdocumentation. Being able to decrease inter-observer andof diabetic retinopathy grading. Ultra-widefield imaging andintra-observer variability can prevent disagreement in clinicalclinical examination agreed exactly in 70% (137) of eyes andmanagement, especially when a patient is seen by multipleagreed within 1 level of diabetic retinopathy in 93% (184) ofdoctors within a practice which improves patient outcomeseyes.1 These findings are even more impressive consideringand reduces patient anxiety and frustration. that ultra-widefield imaging was performed more quickly thanstandard photography and without the need for dilation. Recentinvestigations on the use of ultra-widefield imaging in diabeticretinopathy have shown that the presence of peripheral diabeticretinopathy is associated with a 4.7-fold increased risk ofprogression to PDR and a 3.2-fold increased risk of progressionin diabetic severity by at least 2 levels2. A surprising result tocome out of this study is that 51% of all patients had diabeticretinopathy lesions that were predominantly peripheral.Apart from its utility in pathology detection, ultra-widefieldimaging is becoming an essential part of many practices as thehealthcare landscape is quickly changing. As eye care providersare being asked to see more patients, while providing qualitycare in less time with shrinking reimbursement, technologiessuch as auto-refractors and optical coherence tomography(OCT) have become indispensable. OCT has revolutionizedophthalmic care by improving detection and assessment ofocular disease while auto-refractors have improved doctor1A 200 ultra-widefield image of a healthy eye.Many would argue that the above mentioned advantages arenot truly evident with current and past iterations of ultrawidefield retinal imaging due to color inaccuracy, retinaldistortion, inadequate image resolution, or difficulty in image
acquisition. The latest instrument from ZEISS, the CLARUS 500,The ZEISS CLARUS 500 combines an easy image acquisitionaims to solve all of these issues and provide quality imagesplatform with an intuitive user interface to allow for thethat are easily acquired. It is a true color ultra-widefield retinaleffortless review of images. Sequential imaging of lesions isimaging device that provides high-quality images of the opticsimplified by pulling forward previous image annotations.nerve head, macula, and periphery with 7 micron resolution. ToEvaluation of several images at once, including different colorhelp in the disease management of both macular and peripheralchannels and FAF, is streamlined by coordinating images to thedisease, the ZEISS CLARUS 500 is able to take 133 true colorsame retinal locus and zoom. The image review software can beimages with one capture or 200 images with an auto-mergeinstalled on multiple computers to improve office flow.feature. Stereoscopic photography and fundus autofluorescence(FAF) provide additional pathology visualization and detectionCurrently, many practitioners have a standard fundus camera tocapabilities. The ZEISS CLARUS 500 uses three wide-spectrumhelp in the management of posterior pole disease and an ultra-LEDs to enable image capturing in true color and reduce opticwidefield system for the management of peripheral disease.nerve head bleaching. The partially confocal optics reduce lid,Due to its increased resolution and true color, the ZEISS CLARUSlash, and other artifacts from the anterior segment. External500 may aid in detection of both posterior pole disease such asocular images can also be acquired to aid in anterior segmentglaucoma and peripheral disease such as lattice degeneration ordisease management and education. The ZEISS CLARUS 500retinal tears. These images are similar to what we traditionallycombines numerous technical features into a sleekly designedsee during our clinical exam, decreasing the learning curve inplatform with both patient and operator comfort in mind. Itimage interpretation and disease detection. Unlike previousutilizes a standard slit lamp/joystick approach and a chin restsystems, the ZEISS CLARUS 500 also has stereoscopic imagesto improve patient comfort and remove any burden from theto help distinguish pathology in cases where binocularity is anpatient during image acquisition. A live infrared preview of theimportant cue. Due to its vast array of technical features and itsretina assists the operator in obtaining focused images withoutease of use, the ZEISS CLARUS 500 can effectively be the onlythe need for multiple acquisitions. The live infrared preview allowsfundus camera in any office, regardless of whether that office isthe operator to optimize alignment while ensuring that lids anda high volume multi-specialty practice or a one-doctor practice.lashes do not interfere with the image capture, which decreasesimage capture time, eliminates patient exposure to excessiveimage capture flashes, and improves patient experience.A 200 ultra-widefield image of a patient with retinal vasculitisand an external image taken with the CLARUS 500 revealingposterior synechiae.Real and schematic images of the ZEISS CLARUS 500showcasing the patient-centric design.2
ZEISS offers a full suite of diagnostic equipment.The ZEISS CIRRUS HD-OCT provides an invaluabletool for the management of retinal and opticnerve pathology.Similar to the assessment of glaucoma, where multiplediagnostic technologies aid in management, the evaluation ofchoroidal nevi also necessitates a multimodal approach. Fundusphotography is essential for the detection and documentationof choroidal nevus size and pigmentation whereas OCThelps in the evaluation of lesion structure. True color imagescaptured with CLARUS 500 can be separated into red, greenand blue channel images to help enhance the visual contrastA choroidal nevus imaged in color and with blue, red, andgreen color channels. Note how the nevus is best visualizedwith red separation, which best images posterior retinal andchoroidal lesions.of details in certain layers of the retina. True color imaging isalso ideal for discerning abnormalities overlying choroidal nevisuch as yellow drusen and orange lipofuscin. Yellow drusenare most often signs of a benign lesion while the presenceof orange lipofuscin usually denotes a suspicious lesion thatneeds further evaluation. Using the different color channelscan help differentiate choroidal lesions, which are bettervisualized with red separation, from more superficial lesionssuch as CHRPE, which are better imaged with green separation.Using serial fundus photography, any significant changes insize and pigmentation can be easily assessed. These changescan be indicative of a choroidal melanoma and would requirefurther evaluation. Infrared imaging and FAF can help visualizelipofuscin or retinal pigment epithelium (RPE) dysfunction,which may be suggestive of a metabolically active lesion such asa small choroidal melanoma. When reviewing multiple images,the ZEISS CLARUS 500 image review software can coordinatethe images to the nevus using a common zoom factor. Onecan easily review color and FAF images from multiple dates toevaluate small changes in lesion size, color, or FAF. Annotationssuch as size are propagated onto future images so that repeatmeasurements do not need to be made. Other risk factors forchoroidal melanoma, such as lesion thickness and presenceof subretinal fluid, are more readily evaluated with OCT. Thismultimodal approach to nevus evaluation can help preventsuperfluous referrals and preventable melanoma growth.A choroidal nevus imaged with widefield FAF and OCT. FAF isan RPE phenomenon; therefore, the nevus is not visualized withFAF because it is posterior to the RPE.Central serous chorioretinopathy (CSC) lends itself well tomultimodal imaging due to the significant variability in clinicalpresentation, especially between the acute, chronic, andresolved forms of the disease. RPE atrophy can occur evenafter a relatively quick self-limiting case of CSC. These changescan be visualized with B-Scan OCT as mild degenerations ofthe RPE, but are more readily visualized with FAF. Areas ofhypoautofluorescence represent areas of RPE atrophy whilehyperautofluorescence denotes areas of metabolically stressedRPE that often times convert to atrophic regions. As opposedto the classic symptomatic central serous retinal detachment,CSC may also present extra-foveally without any reportedsymptoms. These cases are often incidental findings and maypresent as scattered RPE changes resulting from resolved serousdetachments. The CLARUS 500 may be particularly useful inthese cases because it combines ultra-widefield imaging withFAF to provide sweeping views of RPE dysfunction that is often3understated with standard fundus photography or DFE.
OCT B-scan images through three retinal loci showing varyingamounts of outer retinal disease.A serous retinal detachment secondary to CSC is noted withfundus photography and OCT.Widefield FAF of the fellow eye reveals mild hypo-FAFchanges inferior-nasal to the foveal that are suggestive ofretinal atrophy. Widefield FAF is preferred in these type ofcases because these mild changes are often invisible toophthalmoscopy and fundus photography.Though diabetic retinopathy is classically thought to be aMultifocal retinal atrophy secondary to resolved CSC changesnoted with color photography and widefield FAF. FAF revealshyper-FAF, which is thought to indicate a metabolically active or“sick RPE.” The hypo-FAF areas represent signs of retinal atrophy.Note the area of RNFL myelination superior to the optic nervehead which masks the FAF signal causing relative hypo-FAF.posterior pole disease, as mentioned above, recent studies haveshown that hemorrhaging is frequently found peripherally aswell. The ZEISS CLARUS 500 can image peripheral hemorrhagingbut also provide high-quality images of the macula. Theseimages can be zoomed in to evaluate the fovea for smallmicroaneurysms. Serial high-quality images of the macula shouldbe assessed for high microaneurysm turnover, as this has beenshown to have a high-risk association with the developmentof diabetic macular edema3. The presence of hemorrhagesand cotton wool spots is important to document but the mostcommon cause of vision loss in these patients is diabetic macularedema, which is significantly harder to document with fundusphotography. In cases of suspected diabetic macular edema,OCT imaging can definitively rule out the presence of retinalthickening and prevent unnecessary referrals.4
Widefield color image of diabetic retinopathy with exudation.OCT imaging confirms the exudation though there is nosignificant associated retinal thickening.Widefield color imaging documents chorioretinal atrophy andwidefield FAF imaging reveals hyper-FAF changes surroundinghypo-FAF changes, suggestive of an ongoing pathologicalprocess. Grayish lesions temporal the macular are vitrealopacities, likely secondary to a prior episode of vitritis.Widefield FAF imaging of the fellow eye reveals mild changesthat were invisible to ophthalmoscopy and color imaging.Widefield color images reveal diabetic retinopathy withexudation. On the left, anti-VEGF can be seen floatingsuperiorly within the vitreous. OCT imaging confirms exudationand mild retinal thickening.Chorioretinal scarring can create a diagnostic predicamentfor the eye care practitioner. Often times, the etiology of achorioretinal scar is unknown and if the lesion looks inactive,not much concern is warranted. Without associated findingssuch as active uveitis, active vitritis, or hemorrhaging,chorioretinal scars are often monitored yearly. Using FAFcapabilities, seemingly inactive lesions may appear to bemetabolically active and perhaps require closer follow up.Because of its wide scan size, The ZEISS CLARUS 500 mayvisualize other lesions within the same eye or within the felloweye that are difficult to visualize with DFE or color photography.Disseminated or bilateral retinal findings may change not onlythe prognosis but follow up and management of these patients.The differentiation of optic nerve head drusen from optic nervehead edema can often be a difficult clinical decision. Superficialoptic nerve head drusen may be easy to distinguish with DFEor fundus photography but buried optic nerve head drusen areoften difficult to appreciate. Unlike standard fundus photography,which has minimal utility in the differentiation of buried discdrusen from papilledema, FAF imaging can greatly aid in thevisualization of buried disc drusen. Though devoid of lipofuscin,optic nerve head drusen are significantly hyperautofluorescentand easily imaged with FAF. Once identified, optic nerve headdrusen must be monitored regularly due to possible compressionof the RNFL and resultant atrophy that is very similar toglaucoma. Arcuate defects on visual field and RNFL thinning onOCT may occur even though there may be minimal cupping.4As the atrophy progresses, optic nerve head pallor and nonglaucomatous cupping may appear. This progression can bemonitored with OCT RNFL imaging, OCT ganglion cell analysis,and visual field testing. Though no accepted treatment for opticnerve head drusen exists at this time, some practitioners initiateocular hypotensive treatment to prevent further damage.5
References1 Silva, Paolo S., et al. “Nonmydriatic ultrawide field retinal imagingcompared with dilated standard 7-field 35-mm photography andretinal specialist examination for evaluation of diabetic retinopathy.”American journal of ophthalmology 154.3 (2012): 549-559.2 Silva, Paolo S., et al. “Peripheral lesions identified on ultrawide fieldimaging predict increased risk of diabetic retinopathy progressionover 4 years.” Ophthalmology 122.5 (2015): 949-956.3 Nunes, Sandrina, et al. “Microaneurysm turnover is a biomarkerfor diabetic retinopathy progression to clinically significantmacular edema: findings for type 2 diabetics with nonproliferativeretinopathy.” Ophthalmologica 223.5 (2009): 292-297.The optic nerve head drusen are not easily visualized with colorimaging though a corresponding retinal nerve fiber layer is notedsuperior-temporally. FAF imaging easily visualizes the optic nervehead drusen, noted as discrete hyper-FAF round lesions.Eye care practitioners are no longer managing retinal and opticnerve head disease based merely on appearance, but are usingcutting-edge approaches to assess structure and function in amyriad of ways. The ZEISS CLARUS acquires high-quality, truecolor and FAF images of the posterior pole and retinal periphery.Together with OCT, OCTA and visual field testing, ZEISS offersa comprehensive suite of diagnostic devices to aid in detectionand diagnosis of the full spectrum of ophthalmic diseases.In a world of decreasing reimbursement and busier patientschedules, the ZEISS CLARUS 500 aims to fulfill whatultra-widefield technology initially promised: high-qualityimaging that improves patient care and office flow.64 Auw-Haedrich, Claudia, Flemming Staubach, and Heinrich Witschel.“Optic disk drusen.” Survey of ophthalmology 47.6 (2002): 515-532.CAM.10092 www.zeiss.com/us/medThe statements of the doctor who has written this white paper reflect only their personal opinions and experiences and do notnecessarily reflect the opinions of any institution with whom they are affiliated. The doctor who has written this white paper hasa contractual relationship with Carl Zeiss Meditec, Inc., and has received financial compensation. 2018 Carl Zeiss Meditec, Inc. All rights reserved. Only for sale in selected countries.Numerous optic nerve head drusen are easily visualized withcolor photography. The optic nerve head drusen are noted asdiscrete areas of hyper-FAF.
ease of use, the ZEISS CLARUS 500 can effectively be the only fundus camera in any office, regardless of whether that office is a high volume multi-specialty practice or a one-doctor practice. A 200 ultra-widefield image of a patient with retinal vasculitis and an external image taken with the CLARUS 500 revealing posterior synechiae.










