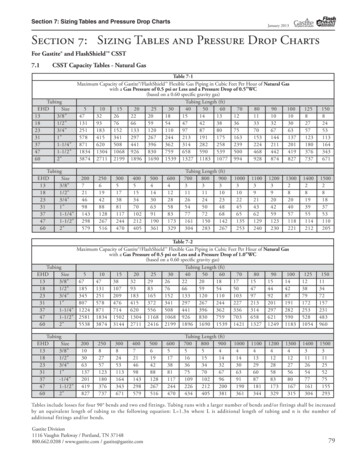
Transcription
Section 3 - Measurements, Scales, and Conversions1 of 8Read the following notes on recording measurements, choosing the correct scale, and convertingmeasurements. It may help to underline, highlight, or make notes in the margin of this documentas you study. Answer the questions to follow.In the world of science, we use the metric system to record measurements of solids, liquids, andgases. The metric system is easy to follow, mainly because it uses units with divisions that arepowers of ten. Using the same system worldwide ensures that any scientist in the world canrepeat the experiment of another easily, without less room for error when converting units. Themetric system is often abbreviated using the letters SI, which stands for System International(derived from the French).In the metric system, we use the following base units:Mass is measured in GRAMS, using a triple beam balance or scale.Volume is measured in LITERS, using a graduated cylinder for liquids.Distance is measured in METERS, using a ruler or meter stick.Temperature is measured in degrees CELSIUS, using a thermometer.Measuring Mass with a Triple-Beam BalanceA triple-beam balance has a pan and three beams with sliding masses called riders. At one end ofthe beams is a pointer that indicates whether the mass on the pan is equal to the masses shown onthe beam.To use:1. Make sure the balance is zeroed before measuring the mass of an object. The balance is zeroedif the pointer is at zero when nothing is on the pan and the riders are at their zero points.2. Place the object to be measured on the pan.3. Move the riders one notch at a time away from the pan. Begin with the largest rider (usually100 g). If moving the rider one notch brings the pointer below zero, begin measuring the masswith the next smallest rider.4. Change the positions of there ideas until they balance the mass on the pan and the pointer is atzero. Then add the readings from the three beams to determine the mass of the object.For example, the image above shows masses of 20g, 0g, and 7.70g, giving a total mass of27.70g.
Section 3 - Measurements, Scales, and Conversions2 of 8Tips:1. When using a weighing boat or weighing paper, be sure to zero the balance after you’veplaced the boat or paper on the pan and before your add your substance to the boat or paper.2. Remember that the riders may not be in order by mass. For example, the image on theprevious page shows mass increments of 10g on the top beam, 100g on the middle beam, and 1gon the bottom beam.3. Some balances may have more than three beams.4. We can only estimate the last digit of the measurement, unless it appears to be exact. Forexample, the measurement 45.62 g gives 45.6 grams with certainty, with 0.02 g estimated by thereader.Measuring Volume using a Graduated CylinderTo use:1. Be sure to have your eyes at the level of the surface of theliquid when reading the scale on the graduated cylinder.2. The surface of most liquids will be curved slightly down whenthey are held in a graduated cylinder. This curve is called themeniscus. Read the volume of the liquid at the bottom of themeniscus, as shown.3. The volume will often be between two lines on the graduatedcylinder. You should estimate the final digit in your measurement.For example, if the bottom of the meniscus appears to be exactlyhalf way between the marks for 96 mL and 97 mL, you wouldread a volume of 96.5 mL.4. To find the volume of a small solid object, record the volumeof some water in a graduated cylinder. Then, measure the volumeof the water after you add the object to the cylinder. The volume of the object is the differencebetween the first and second measurements. This is called the water displacement method.Tip:Do not use a beaker to measure the volume of a liquid. Beakers are used for holding and pouringliquids. To avoid overflow, be sure to use a beaker that holds roughly twice as much liquid as youneed.Measuring Temperature using a ThermometerThe thermometers you will be using measure temperature in degrees Celsius ( C). Each divisionon the scale represents 1 C. The average human body temperature is 37 C. A typical roomtemperature is between 20 C and 25 C. The freezing point of water is 0 C and the boiling pointof water is 100 C.Electronic thermometers, often called temperature probes, are used to record temperature over arange of time or to give more accurate and precise readings.
Section 3 - Measurements, Scales, and Conversions3 of 8To use:1. Place the thermometer in your sample and wait for 30 seconds before taking thereading.2. Be sure to have your eyes at the level of the surface of the liquid when reading thescale.3. The temperature will often fall between two lines on the thermometer. You shouldestimate the final digit in your measurement.4. Do not touch the sides or bottom of your container with the thermometer. This canyield a false temperature.For example, the thermometer at right shows a measurement between 55 C and 60 C.We could estimate the reading to be 57 C.Measuring Length using a Ruler or MeterstickUse a metric ruler or meterstick to measure the length of an object. On the ruler, each markednumber represents one centimeter (cm). The smaller lines between each centimeter representmillimeters (mm). There are 10 mm in one centimeter and 100 cm in one meter (m).To use:1. Place the metric ruler so that the 0-cm mark lines up with the end of the object.2. Be sure to have your eyes at the level of the object when reading the scale on the ruler.3. You may only estimate the final digit of your measurement.Accuracy, Precision, and ErrorAccuracy refers to how close a measurement is to the real value or the accepted value.Precision refers to how close a series of measurements are to one another, or to what degree ameasurement can be recorded.Accuracy is generally affected by the user’s setup and technique when recording measurements.For example, failing to zero a triple-beam balance, or failing to record temperature or volume ateye level would all yield inaccurate measurements.
Section 3 - Measurements, Scales, and Conversions4 of 8Precision is generally affected by the degree to which a tool can measure. For example, a rulerthat measures to the nearest centimeter is less precise than a ruler that measures to the nearestmillimeter. In addition, precision is decreased when multiple measurements yield differentresults.Some examples:Suppose you were to weigh yourself using a scale that is not zeroed. You weigh yourself 10times, and each time, you record your weight as 65 kg. Though your measurements are precise,they are inaccurate because the scale was not zeroed first. The same would happen if we were toconsistently measure a graduated cylinder 20 above eye level, rather than at eye level, or if weused a ruler but did not line up the object at the zero mark. These situations both display usererror or poor technique - the reader is not using the tool accurately.In contrast, suppose you were to measure the mass an object using a triple-beam balance. Eachtime, you zero the balance before recording the measurement. However, your five measurementshave masses of 25.5 g, 25.7 g, 24.9 g, 25.5 g, and 25.3 g. The known mass of the object is 25.5grams. In this example, because the results you obtained are not consistent, you could describeyour process and measurements as fairly accurate, but they are not precise.Oftentimes, if a measurement is inaccurate, it’s because of user or operator error (poor techniqueby the scientist). If a measurement is not precise, it is often because of a limitation on the tool’spart (it doesn’t measure to a greater decimal place, or it doesn’t provide consistent results).VolumeVolume is the amount of space contained in an object. We can find the volume of box shapes byusing the formula volume length x width x height.DensityDensity is the amount of matter (mass) compared to the amount of space (volume) an object has.Density can be calculated using the formula density mass/volume.Since the unit for mass is grams, and the unit for volume is mL or cm3, then the unit for densityis often g/mL, or g/cm3.Water has a density equal to 1g/mL.Objects with a density less than 1g/mL will float on top of water. Objects with a density greaterthan 1g/mL will sink in water.
5 of 8Section 3 - Measurements, Scales, and ConversionsPrefixesWhen recording measurements, we can use the following prefixes, which represent the numbers(or place holders) below:PrefixAbbreviati Power ofonTenFactor of Base 1,000Hectoh102100Dekada10110BASEm, g, or 000,000,000,001For example, 1 km is equal to 1000 m, and 15 dg are equal to 1.5 centigrams.When converting one prefix to another, it is helpful to remember the six primary prefixes withthe following pneumonic phrase:King Henryfor the prefixeskilo- nti-milli-Unit ConversionsSometimes it is beneficial to use one particular prefix over another. In these cases, a unitconversion may be required. Using SI units and prefixes, one unit can easily be converted toanother. The easiest way to do this is to move the same number of decimal places to the right orleft.For example, suppose you are asked to record 1540 m in km. Using the list above, you see thatbeginning with your base unit (meters), kilo- is located 3 spaces to the left. In your initialmeasurement, 1540, it is implied that the decimal place exists after the zero (1540.). Since kilois 3 moves to the left, we simply move the decimal place 3 spaces to the left, giving us 1.540 km.
Section 3 - Measurements, Scales, and Conversions6 of 8Another way to look at this is to remember that kilo- represents 1000. To convert 1540 m to km,we must remember that there are 1000 m in 1 km. Thus,1540 m x1 km 1.540 kmor1540 m / 1000 1.540 km1000 mAnswer the following questions on a separate sheet of paper. Remember to use the followingformatting:- If typed, use size 12 Times New Roman font and single spacing.- If handwritten, print your responses neatly.- Leave one line of blank space between each response.- Use the following heading in the top left corner of your response sheet:Student NamePre-AP BiologySummer Assignment, Section #Date of completion (not the due date)Section 3, Part I Review Questions: You do not have to use complete sentences.1. Which base units would you use to record the following measurements?a. the volume of a swimming poolc. the temperature on a sunny dayb. the mass of your dogd. the length of a paper clip2. Identify the following masses shown on the balances. Be sure to include the appropriate unitsof measure.a.b.c.
Section 3 - Measurements, Scales, and Conversions7 of 83. Identify the following volumes shown on the graduated cylinders. Be sure to include theappropriate units of measure.a.b.c.4. Identify the following temperatures shown on the thermometers. Be sure to include theappropriate units of measure.a.b.c.5. Identify the following lengths shown on the rulers. Be sure to include the appropriate units ofmeasure.a.b.
Section 3 - Measurements, Scales, and Conversions8 of 8Section 3, Part II Review Questions: Use complete sentences for #1-4.1. Describe the difference between accuracy and precision.2. Provide a situation in which a measurement may be accurate, but is not precise.3. Provide a situation in which a measurement may be precise, but is not accurate.4. What should we look for to be certain a measurement is both accurate and precise?5. Convert 110 cm to dm.6. Convert 4567 g to kg.7. Convert 399.57 L to mL.8. Convert 986.42 hg to kg.9. Convert 8.47 hL to daL.10. Convert 0.342 g to cg.There will be a quiz on measurements and conversions during the first week of school. Youwill also be asked to practice and demonstrate proper use of the measuring tools described inthis packet.
they are held in a graduated cylinder. This curve is called the meniscus. Read the volume of the liquid at the bottom of the meniscus, as shown. 3. The volume will often be between two lines on the graduated cylinder. You should estimate the final digit in your measurement. For example, if the bottom of the meniscus appears to be exactly half way between the marks for 96 mL and 97 mL, you .