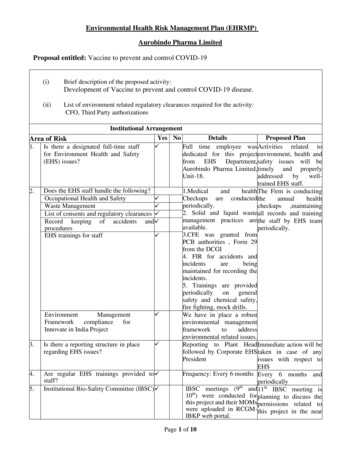
Transcription
StandardOperatingProcedureBIOMEDICALWASTE INHALTHCAREFACILITIESAugust, 2020Page 0 of 22
STANDARD OPERATING PROCEDURESBIOMEDICAL WASTE MANAGEMENT IN HEALTHCARE FACILITIESAUGUST 2020Page 1 of 22
Contents1.BACKGROUND . 32.THE OBJECTIVES OF THE PROGRAM . 33.Health care waste – Introduction . 34.5.3.1Health care waste . 33.2Health care waste management system . 63.3Key responsibilities . 7STANDARD OPERATING PROCEDURES FOR BIOMEDICAL WASTE MANAGEMENT IN HEALTHCARE FACILITIES74.1Situation analysis . 94.2Establish benchmarks/ standards, and develop action plan . 104.3Improve and maintain the BWM infrastructure and facilities – Action plan . 184.4Monitor and review . 20PREREQUISITES AND PREPARATIONS . 215.1Policy framework . 215.2Institutions . 225.3Materials and equipment . 225.4Capacities, training and Human resource . 225.5Finances . 22Page 2 of 22
1. BACKGROUNDCYDA in collaboration with UNICEF in an effort to improve WASH Access in 19 health facilitiesand 50 community toilets in slums under the Pune Municipal Corporation areas” has been engaged. It is related to Bio Medical waste and Water, Sanitation and Hygiene in Hospital and 11Hospitals, 8 dispensaries and 50 selected community toilets in PMC area. We have list of 11 hospital and 8 dispensaries for assessment of BMW and WASH in Health Facilities in 11 hospitals & 8dispensaries under PMC. The project will look into the gender perspectives to ensure access anduse of WASH facilities in the community and health facilities. Children, adolescents and womenare most affected among all during this situation. The project will be implemented in the Government Facilities targeting patients irrespective of class, gender and age disaggregation, etc.The access to these facilities is free or with a very nominal fees as these are run by the PMC –Urban Local Body. These hospitals and dispensaries are spread across the city and already focusses on equity and access. An effort will also be made to sensitize the staff of the HCFs on genderequity and access through interactions, orientation and bringing in the elements in the SoPs andchecklists.2. THE OBJECTIVES OF THE PROGRAM To strengthen biomedical waste management mechanism in 11 hospitals and 8 dispensaries under the PMCTo support for ensuring clean environment in the selected healthcare facilities with adequate WASH facilities.To make available SoPs and Checklists for hospital staff, ward officers, CBOs and toiletoperators to build resilience around Covid19 and the Corona virus.To build the capacities of the stakeholders under this initiative in PMC area.To demonstrate a WASH model for healthcare facilities and community toilets in theslums of PMC and for wider dissemination.To leverage resources for sustaining and replicating the demonstrated model across thePMC area.3. Health care waste – Introduction3.1 Health care wasteBiomedical waste as the name indicates include waste generated from the health care facilities.Health Care Facilities are responsible for management of the healthcare waste generated withinPage 3 of 22
the facilities, including activities undertaken by them in the community. Bio-medical waste include any waste that is in health care facilities during various health treatment such as diagnosis,treatment, immunization and related activities. If not disposed in safe manner; it can cause detrimental impacts on human health and environment.Waste generated from the healthcare facility – which can be called as health care waste, include– biomedical waste, general waste and other waste.a) Biomedical waste can be infectious if the source of such waste is from infectious personand if such waste in not properly handled and disposed. As per the Bio Medical WasteManagement Rules, 2016; the bio-medical waste has been categorized into Yellow, Red,White, and Blue. These categories are further divided as per the type of waste undereach category as follows:Table 1: Categories of Biomedical WasteBiomedical Waste TypeCategoryYellowHuman Anatomical WasteHuman tissues, organs, body parts and fetus below the viability period (as per theMedical Termination of Pregnancy Act 1971, amended from time to time).Animal Anatomical Waste Experimental animal carcasses, body parts, organs, tissues, including the waste generated from animals used in experiments or testing in veterinary hospitals or colleges or animal houses.Soiled WasteItems contaminated with blood, body fluids like dressings, plaster casts, cottonswabs and bags containing residual or discarded blood and blood components.Discarded or Expired MedicinePharmaceutical waste like antibiotics, cytotoxic drugs including all items contaminated with cytotoxic drugs along with glass or plastic ampoules, vials etc.Chemical WasteChemicals used in production of biological and used ordiscarded disinfectantsChemical Liquid WasteLiquid waste generated due to use of chemicals in production of biological andused or discarded disinfectants, Silver X – ray film developing liquid, discardedPage 4 of 22
Formalin, infected secretions, aspirated body fluids , liquid from laboratoriesan d floor washings, cleaning, house - keeping and disinfecting activities, etc.Discarded linen, mattresses, beddings contaminated with blood or body fluid, routine mask & gown.Microbiology, Biotechnology and other clinical laboratory waste (Pre-treated)Microbiology, Biotechnology and other clinical laboratory waste: Blood bags, Laboratory cultures, stocks or specimens of microorganisms, live or attenuated vaccines, human and animal cell cultures used in research, industrial laboratories,production of biological, residual toxins, dishes and devices used for cultures.RedWastes generated from disposable items such as tubing, bottles, intravenoustubes and sets, catheters, urine bags, syringes without needles, fixed needlesyringes with their needles cut, vaccutainers and glovesWhileWaste Sharps including metalsNeedles, syringes with fixed needles, needles from needle tip cutter or burner,scalpels, blades, or any other contaminated sharp object that may cause punctureand cuts. This includes both used, discarded and contaminated metal sharpsBlueBroken or discarded and contaminated glass including medicine vials and ampoules except those contaminated with cytotoxic wastes.All such waste which can adversely harm the environment or health of a person is considered asinfectious and such waste has to be managed as per BMWM Rules, 2016.b) General WasteGeneral waste consists of all the waste other than biomedical waste and which has notbeen in contact with any hazardous or infectious, chemical or biological secretions anddoes not includes any waste sharps. These general wastes are further classified as drywastes and wet wastes and should be collected separately.The quantity of general waste is around 85 % to 90 % of total waste generated. Generalwaste is required to be handled as per the Solid Waste Management Rules, 2016 andConstruction & Demolition Waste Management Rules, 2016, as applicable. This wasteconsists of mainly:i.Organic / Bio-degradable waste - mostly food waste (wet waste)ii.Food Containers after emptying residual food (dry waste)Page 5 of 22
iii.News paper, paper and card boxes (dry waste)iv.Plastic water bottles (dry waste)v.Aluminum cans of soft drinks (dry waste)vi.Packaging materials (dry waste)vii.Construction and Demolition wastesc) Other WastesOther wastes consist of used electronic wastes, used batteries, and radio-active wasteswhich are not covered under biomedical wastes but have to be disposed as and whensuch wastes are generated as per the provisions laid down under E-Waste (Management)Rules, 2016, Batteries (Management & Handling) Rules, 2001, and Rules/guidelines underAtomic Energy Act, 1962 respectively.3.2 Health care waste management systemDirectorate General of Health Services Ministry of Health & Family Welfare and Central Pollution Control Board (CPCB) have issued specific guidelines for managing healthcare waste asper the Biomedical Waste Management Rules, 2016. Following these broader guidelines, aset of standard operating procedures have been recommended, so that the rules, guidelinesare implemented in the healthcare facilities.The entire healthcare biomedical waste management system include the following elementsand steps - segregation, Collection, pre-treatment, Intramural Transportation, Storage,Treatment and Disposal:-Figure 1: Elements of Biomedical Waste management systemPage 6 of 22
3.3 Key responsibilitiesBased on the type of element sand work, the responsibilities have been assigned. Accordingly, segregation, Collection, pre-treatment, Intramural Transportation and Storage) is theresponsibility of Health Care Facility.While Treatment and Disposal is primarily the responsibility of Common Biomedical WasteTreatment Facility (CBWTF) operator.Lab and highly infectious waste, is required to be pre-treated by the Health Care Facility.Following are the responsibility of HCF for management and handling of bio-medical waste:1. Segregate Biomedical Waste should at source (the point of generation) by theperson who is generating the waste in the designated colour coded bin/ container2. Biomedical Waste & General Waste shall not be mixed.3. Storage time of waste should be as less as possible so that waste storage, transportation and disposal is done within 48 hours.4. Phase out use of chlorinated plastic bags (excluding blood bags) and gloves by27/3/2019.5. No secondary handling or pilferage of waste shall be done at healthcare facility.6. If CBWTF facility is available at a distance of 75 km from the HCF, bio-medicalwaste should be treated and disposed only through such CBWTF operator.7. Only Laboratory and Highly infectious waste shall be pre-treated onsite beforesending for final treatment or disposal through a CBWTF Operator.8. Provide bar-code labels on all colour coded bags or containers containing segregated bio-medical waste before such waste goes for final disposal through aCBWTF.4. STANDARD OPERATING PROCEDURES FOR BIOMEDICAL WASTEMANAGEMENT IN HEALTHCARE FACILITIESIn order to effective handle and manage waste from healthcare facilities a set of procedureshave been recommended as given below.Page 7 of 22
Accordingly, the following standard procedures or standard operating procedure (SOPs) havebeen recommended to ensure effective management of Biomedical waste, general waste andother waste generated in the healthcare facilities.1. Understand the Situation of health care waste management - Situation analysis2. Establish benchmarks and standards3. Defining targets and developing Action plan considering stakeholder engagement4. Improving and maintain waste management system in the healthcare facilities includingstrengthen workforce engaged in waste management5. Monitoring and review6. Share learningsSituationanalysisEstablishbenchmarksand standardsDefining targetsand developingAction planShare learningsMonitor andreviewImprove andmaintain WASHfacilitiesPage 8 of 22
4.1 Situation analysisSituational analysis of the existing waste management system and levels of services availablehas to be carried out. Situational analysis must be carried out covering all the aspects thatwe wish to improved based on different applicable waste management Rules. Accordingly, afollowing set of parameters have to be assessed.1. Steps involved in Bio-medical Waste Management2. Status of Bio Medical Waste Segregation2.1 Color Coding system adopted and Type of Container/ Bags to be used for Waste Segregation & Collection3. Status of Bio Medical Waste Collection3.1 Time of Collection3.2 Packaging3.3 Labeling3.4 Interim Storage3.5 Arrangement of in-house Transportation of Bio Medical Waste3.6 Route of intramural transportation of bio-medical waste3.7 Central Waste Collection Room for Bio-medical Waste4. SEGREGATION, TREATMENT AND DISPOSAL OF BMW4.1 Status of segregation of different waste types4.2 Existing Provisions of treatment4.3 Existing Disposal practices4.4 Existing Standards for Treatment and Disposal as per BMWM Rules, 20165. Status of BMW MANAGEMENT AT OUTREACH ACTIVITIES AND BY OCCASIONAL GENERATORS6. Existing role and capacities of the staff and management of the Health Care Facility7. Responsibilities versus delivery of various staff8. Health status of workers engaged in waste management8.1 Personal Protection measures taken by various staffPage 9 of 22
9. Provisions and Status of OCVID waste management10. Records and data management11. Finances and budget allocations versus spending and its utilization12. Information Education and Communication13. Existing Operation, maintenance and Management14. Existing Monitoring, Evaluation and review Mechanism4.2 Establish benchmarks/ standards, and develop action plan Benchmarks, standards for health care waste management in healthcare facilities have tobe defined – The Table below defines these standards based on the expectations and existing protocols and rules Based on these standards, targets for each of the parameters have to be defined alongwith the timelines; so that the standards and benchmarks could be achieved within a defined timeframe Based on the defined targets – an action plan for its implementation has to be developedTable 2: Benchmarks/ standards for health care waste management in healthcare facilitiesParametersStandards1. Steps involved in Bio-medical Pre-treatmentIntramural TransportationStorageTreatment andDisposal2. Bio Medical Waste Segregation2.1 Color Coding system adoptedand Type of Container/ Bags tobe used for Waste Segregation &Yellow, Red, While, Blue as described in Table3.Page 10 of 22
ParametersStandardsCollection3. Bio Medical Waste Collection system3.1 Time of Collection3.2 Packaging-3.3 Labeling-3.4 Interim Storage-3.5 Arrangement of in-house Transportation of Bio Medical Waste-3.6 Central Waste Collection Roomfor Bio-medical Waste-Daily, fixed time¾ bag filled, sealed, no staple, colour codingWith the Symbol of Bio Hazard or CytotoxicHazard as the case may beBio-medical waste bags / containers arerequired to be provided with bar code labels in accordance with CPCB guidelinesAvoid as far as possible. If needed storeonly in designated placeSpecial Transportation TrolleysDefined & safe route of intramural transportation of bio-medical wasteA well facilitated Central Waste CollectionRoomCentral Storage for HCFs Having CaptiveTreatment and Disposal SystemMaintain records - category wise generation, its treatment, disposal on daily basis.As per Box 1.3.7 Record Keeping3.8 Updating of Information inWebsite4. Segregation, Treatment And Disposal4.1 Segregation of different wastetypes4.2 Provisions of treatment4.3 Disposal practices5. BMW Management at Outreach ActivitiesAs described in Table 3A full proof and well-designed system for asdescribed in Table 4i. Segregate biomedical waste at the pointof generation i.e. during the outreachPage 11 of 22
ParametersStandardsactivityii. Collection and packaging of waste incolor coded and bar code labelledbags/containersiii. Transportation of waste from outreachactivity site to HCF or make arrangement with nearby CBWTF to collect thewaste directly after completion of outreach activity.iv. Treatment & disposal at HCF or CBWTF6. BMW Management by OccasionalGeneratorsi.ii.iii.iv.v.vi.vii.7. Role and capacities of the staff andmanagement of the Health Care Fa-Obtain one-time authorization underBMWM Rules, 2016 from the prescribedauthority;Obtain agreement with the CBWTF operator for final treatment and disposalof bio-medical waste;Inform CBWTF operator to pick-up biomedical waste as and when it is generated;Segregate the bio-medical waste as percolour coded categories stipulated under BMWM Rules, 2016;The colour coded bags/containersshould be labelled with bar code label(provided by the operator of CBWTF orany authorised vendor).It shall be ensured that anatomicalwaste, soiled waste and biotechnologywaste if generated is treated & disposedwithin 48 hours.Maintain record pertains to quantum ofcategory wise bio-medical waste generated and treated.All staff working with the healthcare facilities –directly and indirectly - capacity to be built inPage 12 of 22
ParameterscilityStandardsall aspects8. Health status of workers engaged inwaste managementAll Staff is provided with Personal Protectionequipment9. Provisions of COVID waste managementTo be made managed along with this existingBiomedical waste10. Records and data managementData on all parameters covered to be recodedand managed11. Information Education and CommunicationExisting, functional and up to date with thecurrent needs12. Operation, maintenance and ManagementExisting and functional13. Monitoring, Evaluation and reviewMechanismExisting and functionalPage 13 of 22
Page 14 of 22
S. No.1.CategoryYellow Category -Table 3: Color Coding system for BMWType of wasteColour & Type of ContainerYellow colored non-chlorinated PlasticHuman Anatomical WasteBagsAnimal Anatomical WasteNote:Soiled Waste(i)Discarded or Expired MedicineChemMicrobiology, Biotechnology andicalother clinical laboratory wastewasteChemical Waste (yellow-e)(yelChemical Liquid Wastelowe) comprising of un-used, residual ordate expired liquid chemicals includingspent hypo of X-Ray, should be stored inyellow containerRed Coloured Non Chlorinated PlasticContaminated Waste (RecyclaBags (having thickness equal to moreble)than 50µ) andContainers2.RedCategory-3.White Category-Waste Sharps including metals(Needles, syringes with fixedneedles, needles from needle tipcutter or burner, scalpels, blades,or any other contaminated sharpobject that may cause punctureand cuts. This includes bothused, discarded and contaminated metal sharps)White Coloured translucent, punctureproof, leak proof, Temper Proof containers4.BlueCategory-Glassware(Broken or discarded and contaminated glass including medicine vials and ampoules exceptthose contaminated with cytotoxic wastes)Metallic Body ImplantsPuncture proof,leak proof boxes orcontainers with-blue colored markingPage 15 of 22
Box 1: Updating of Information in WebsiteAll bedded healthcare facilities should have a separate and updatedpage/web link with the following information:1. Contact Address and details of the Healthcare Facility :2. No. of beds :3. Details of :a) Authorization under BMWM Rules, 2016:b) Consent under Water (Prevention and Control of Pollution)Act, 1974 and Air (Prevention and Control of Pollution)Act, 1981 :4. Quantity of bio-medical waste generation (in kg/day):5. Mode of disposal of bio-medical waste (through CBWTF orthrough captive treatment facility):6. Name and address of the CBWTF through which waste is disposed off (as applicable) :7. In case, HCF is having captive treatment facility,a) bio-medical waste treated (in kg/day)b) Details of treatment equipmentc) Total nos. and capacity of each treatment equipment (inkg/day)d) Operating parameters of the treatment equipment as perBMWM Rules, 20168. Monthly records of bio-medical waste generation (categorywise):9. No. of trainings conducted on Bio-medical Waste Managementin the current year:10.Stats of immunization of Health Care Workers involved inhandling of BMW:Page 16 of 22
S. No.1.CategoryYellow Category-----2.RedCategory-Table 4: Treatment and Disposal OptionsType of wasteTreatment and Disposal OptionsHuman Anatomical- Incineration or Plasma Pyrolysis or deepWasteburialAnimal Anatomical- Incineration or Plasma Pyrolysis or deepWasteburialSoiled Waste- Incineration or Plasma Pyrolysis or deepburial- In absence of above facilities, autoclavingor micro-waving/ hydro laving followedby shredding or mutilation or combination of sterilization and shredding. Treated waste to be sent for energy recoveryDiscarded or Expired- Expired cytotoxic drugs and items conMedicinetaminated with cytotoxic drugs to be returned back to the manufacturer or supplier for incineration at temperature 1200 0C or to common bio-medicalwaste treatment facility or hazardouswaste treatment, storage and disposalfacility for incineration at 12000C OrEncapsulation or Plasma Pyrolysisat 12000C.- All other discarded medicines shall be either sent back to manufacturer or disposed by incineration.Microbiology, Biotech- Pre-treat to sterilize with non-chlorinatednology and other clinichemicals on-site as per National AIDScal laboratory wasteControl Organisation or World Health Organisation guidelines thereafter for Incineration.Chemical Waste (yellow - Disposed of by incineration or Plasma Pycontainers)rolysis or Encapsulation in hazardouswaste treatment, storage and disposalfacility.Chemical Liquid Waste- After resource recovery, the chemicalliquid waste shall be pre-treated beforemixing with other wastewater.- The combined discharge shall conform tothe discharge norms given in Schedule IIIof the BWM Rule 2016.Contaminated Waste- Autoclaving or micro-waving/ hydroclav(Recyclable)ing followed by shredding or mutilationor combination of sterilization andshredding.- Treated waste to be sent to registered orauthorized recyclers or for energy recov-Page 17 of 22
3.White Category-4.BlueCategory--Waste Sharps includingmetals(Needles, syringes withfixed needles, needlesfrom needle tip cutteror burner, scalpels,blades, or any othercontaminated sharp object that may causepuncture and cuts. Thisincludes both used, discarded and contaminated metal sharps)Glassware(Broken or discardedand contaminated glassincluding medicine vialsand ampoules exceptthose contaminatedwith cytotoxic wastes)Metallic Body Implants--ery or plastics to diesel or fuel oil or forroad making, whichever is possible.Plastic waste should not be sent to landfill sites.Autoclaving or Dry Heat Sterilization followed by shredding or mutilation or encapsulation in metal container or cementconcrete; combination of shredding cumautoclaving; and sent for final disposal toiron foundries (having consent to operatefrom the State Pollution Control Boardsor Pollution Control Committees) or sanitary landfill or designated concrete wastesharp pit.Disinfection (by soaking the washed glasswaste after cleaning with detergent andSodium Hypochlorite treatment) orthrough autoclaving or microwaving orhydroclaving and then sent for recycling.4.3 Improve and maintain the BWM infrastructure and facilities – Action planAll the applicable parameters as identified and described in section 4.1 and subsequentsections and the benchmarks defined have to be improved based on the existing situation.Based on the existing situation, benchmarks and standards, action plan has to be developed and implementation of all the proposed interventions have to be taken up. Theaction plan must have: Action plan – based on the gaps identified during situational analysis A definite timeline Defined sources of Finance Provision of Operation and Maintenance Monitoring mechanismPage 18 of 22
Page 19 of 22
4.4 Monitor and reviewPeriodic monitoring of various activities planned is mandatory to ensure that the structuredinterventions are taken up, implemented and quality is maintained.The following Table provides a set of indicators to be used for monitoring and review.Monitoring ParametersObservations/Comments1. All steps involved in Bio-medical Waste Management have beenfollowed as prescribed2. Bio Medical Waste Segregation done at source3. Color Coding system adopted and type of Container/ suggestedbags used for Waste Segregation & Collection4. Bio Medical Waste Collection system with the following details inplace4.1 Time of Collection4.2 Packaging4.3 Labeling4.4 Interim StoragePage 20 of 22
Monitoring ParametersObservations/Comments4.5 Arrangement of in-house Transportation of Bio MedicalWaste4.6 Central Waste Collection Room for Bio-medical Waste4.7 Record Keeping4.8 Updating of Information in Website5. SEGREGATION, TREATMENT AND DISPOSAL system in place5.1 Segregation of different waste types5.2 Provisions of treatment5.3 Disposal practices6. BMW Management at Outreach Activities carried out as recommended7. BMW Management by Occasional Generators carried out asrecommended8. Role and capacities of the staff and management of the HealthCare Facility well defined and built as required9. Health status of workers engaged in waste managementchecked from time to time10. Provisions of COVID waste management made11. Records and data management system available12. Information Education and Communication as needed is carriedout13. Operation, maintenance and Management of the entire systemis carried outThese indicators have to be used to monitor various interventions as needed, with little bitchange as may be needed depending on the needs. Monitoring has to be carried out on daily,weekly, monthly and quarterly basic by internal and external institutions.5. PREREQUISITES AND PREPARATIONS5.1 Policy frameworkA conducive policy framework and supporting guidelines and resolutions is required toenforce BWM Compliance. All the parameters, benchmarks, legal and technical provisions must be made while designing policy guidelinesPage 21 of 22
5.2 InstitutionsInstitutions with adequate technical and other manpower at the facility level are required.There institutions must have a defined duties for their man power considering the needsand requirements.5.3 Materials and equipmentProvision for materials and equipment’s as per the needs assessment based on the situation have to be organized for a well-functioning facility.5.4 Capacities, training and Human resourceCapacities of all the staff, support staff of all institution engaged in the identified facilityhave to build to gear up handling of various identified actions and activities.5.5 FinancesAdequate finances have to be arranged to ensure that the identified actions, activitiesand interventions implemented.Page 22 of 22
STANDARD OPERATING PROCEDURES FOR BIOMEDICAL WASTE MANAGEMENT IN HEALTHCARE FACILITIES In order to effective handle and manage waste from healthcare facilities a set of procedures . Defining targets and developing Action plan considering stakeholder engagement 4. Improving and maintain waste management system in the healthcare facilities .