Transcription
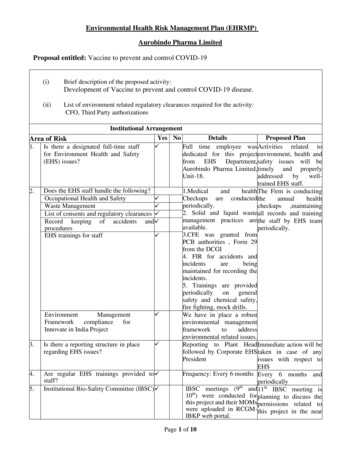
Environmental Health Risk Management Plan (EHRMP)Aurobindo Pharma LimitedProposal entitled: Vaccine to prevent and control COVID-19(i)Brief description of the proposed activity:Development of Vaccine to prevent and control COVID-19 disease.(ii)List of environment related regulatory clearances required for the activity:CFO, Third Party authorizationsInstitutional ArrangementYes NoDetailsProposed PlanArea of Risk 1. Is there a designated full-time staffFull time employee was Activities related tofor Environment Health and Safetydedicated for this project environment, health and(EHS) issues?from EHS Department, safety issues will beAurobindo Pharma Limited, timely and properlyUnit-18.addressedbywelltrained EHS staff.2. Does the EHS staff handle the following?1.Medicalandhealth The Firm is conductingOccupational Health and SafetyCheckups are conducted the annualhealthperiodically.Waste Managementcheckups ,maintaining 2. Solid and liquid waste all records and trainingList of consents and regulatory clearances management practices are the staff by EHS teamRecord keeping of accidents and omEHS trainings for staff PCB authorities , Form 29from the DCGI4. FIR for accidents andincidentsarebeingmaintained for recording theincidents.5. Trainings are providedperiodically on generalsafety and chemical safety,fire fighting, mock drills. EnvironmentManagementWe have in place a robustFrameworkcomplianceforenvironmental managementInnovate in India Projectframeworktoaddressenvironmental related issues. 3. Is there a reporting structure in placeReporting to Plant Head Immediate action will beregarding EHS issues?followed by Corporate EHS taken in case of anyPresidentissues with respect toEHS4. Are regular EHS trainings provided to Frequency: Every 6 months Every 6 months andstaff?periodically5. Institutional Bio-Safety Committee (IBSC) IBSC meetings (9th and 11th IBSC meeting is10th) were conducted for planning to discuss thethis project and their MOMs permissions related towere uploaded in RCGM- this project in the nearIBKP web portal.Page 1 of 10
Environmental Health Risk Management Plan (EHRMP)Aurobindo Pharma Limited6. Ethics Committee (EC)Area of RiskGeneral Occupational Health and SafetyYes No Details7.Are there Standard Operating Procedures for accidents, hazards, and otheremergencies (chemical spills, heat hazards,fire hazards, radioactive hazards etc.)?8.Are the following in place? Chemical spill kits Eye wash Shower stations First Aid Kit Fire Extinguishers Register of accidents and injuries Are proper signage and storage system in place?Display of Material Safety Data Sheet (MSDS) where relevantDisplay of emergency numbers and procedures (Person to Contact, Doctor,Ambulance, Fire Emergency, Police)displayed in all criticalplaces Signage across the facility (labs,storage, hazardous areas, etc.)Are flammable materials appropriately stored to prevent fire hazards?9.10.11.12.Noin-housefacilityAre smoke detectors, fire alarms, automatic safety/shutoffsystems,overflowpreventors, etc. in place and regularlymaintained?Are there control measures for VOC, air emissions, high operating temperatures,pathogens/vectors etc. in place? Are regular mock drills conductedfor emergency preparedness andsafety?future.animalAt present we are nothaving in-house animalfacilityandanimalstudies are out sourcing.In future if required wewill form the EC and willbe intimated.Proposed PlanSOP: Handling of Spillage in ImplementationofViral Vaccine Pilot Plant instructionsasPRD/SOP/130.00mentioned in SOPs toavoid the spillages ofacid, alkali, flammable,chemical, biological andrecombinant materials.All emergency equipment’s Functional and generalare placed at designated maintenance of all safetyplaces to avoid the accidents equipment are regularlyand injuries. Registers of followed. Mock drillsaccidentsandinjuries and safety training weremaintained at Occupational planned periodically toHealth Center (OHC)all the staff members.Emergency signage’s are Emergency contacts willplaced at escape routes and be updated in change ofemergency exits.any key members.Emergency contacts numbers While handling theavailable at security office. flammablematerialsuitable PPE’s are used.MSDS area availableFlammable materials arestored in cool, dry places andaway from the source ofignitionwithadequateventilation.List: MCPS area maintained New MCPS, smokeand smoke detectors area detectorswillbeavailable in all areas inside arranged depending onthe facility.area /sectionList: Stack monitoring is in Done by PCB authorizedplace.third partyFrequency (type wise): 6 MockdrillsareMonthsconductedevery6monthsandarePage 2 of 10
Environmental Health Risk Management Plan (EHRMP)Aurobindo Pharma Limiteddocumented13. Are staff provided with OHS training?Describe: General SafetyAll the staff are providedwith trainings includingnewly joined staff.Biomedical Waste (BMW)Area of Risk14.Yes NoIs there generation of biomedical waste (as described in Bio-Medical WasteManagement Rules, 2016) in thegrantee? 15.Is there trained staff to handlebiomedical waste in the grantee?16.Has the grantee obtained authorization from State Pollution Control Board/Pollution Control Committee?Is the biomedical waste segregated at point of generation in the facility and stored insuitable containers?17.18.Is the bar code system for the segregated waste in place?DetailsProposed PlanIf Yes, provide a list of Biomedical waste will bebiomedicalwaste sent to PCB authorizedproduced in the facility: third party.Decontaminatedcellstacks, Media PlatesIf No, provide a list ofall waste produced in thefacility.Stafftrainedonthe Autoclavedat121decontaminationprocess degrees Centigrade for(Autoclave) and trained on half an hour.the SOP biomedical wastemanagementAuthorization issued by state BMWauthorizationPCBdoneYellow Segregated in yellowRedbags and disposed.WhiteBlueBags segregated with bar Bags are procedure bycode.the approved vendorPage 3 of 10
Environmental Health Risk Management Plan (EHRMP)Aurobindo Pharma Limited19.Is the biomedical waste being sent to an authorized common BMW facility?NameandaddressofCBMWF:M/sDharma & CoBMW will be sent to PCBAuthorized facility everytwo days.Distancefromfacility:120 kmsFrequency andModeoftransport: M/SDharma & Co20Who transports?M/S Dharma &Co Reason:NoSending to PCB authorizedFacilitythird party for disposalswithin 48 hours. Authorization: Distanceofnearest CBWMfrom facility:Does the grantee have an in-house BMWtreatment facility?Is the treatment facility own (individual)?Is the treatment facility a shared facility inan industrial park?212223Are lab waste, microbiological waste andchemical liquid waste pre-treated beforestoring and sending to treatment facilitiesaccording to guidelines prescribed in BWM,2016 regulations?Is the liquid waste checked for activecells before sending to treatment plant? Are necessary waste pre-treatment equipment in place?Do the equipment adhere to prescribednorms by State Pollution Control Board(SPCB)? Typesoftreatment:Typesof After autoclave, lab wastetreatment:will be sent to PCBDecontaminating authorizedpartyforby Autoclavingdisposal for every 48 hours.the materialStandardBefore sending to effluentprocedurestreatment tank the liquidavailablefor wastes is subjected tocheckingactive chemical treatment andcellslater sent to kill tank.Listof Pre-Treatment will be doneequipmentby decontamination by our(autoclaves,staff etailsofwastepretreatment:Kill tankPage 4 of 10
Environmental Health Risk Management Plan (EHRMP)Aurobindo Pharma Limited24.Are chlorinated plastic gloves and bagsphased out in the grantee?25.Are grantee’s personnel involved inhandling BMW provided with regulartraining? For disposal ofAfter packing send tomicrobiology lab authorized PCB authorizedwaste3rd party.Frequency:6 InternalTrainingwasmonthsconducted.Trainer: HOD26.Are medical examination provided to personnel involved in BMW waste handlingand are they providedwith relevantimmunization like Hepatitis B and Tetanus?27.Is a daily register for biomedical wastemaintained including accident reportingrecord?28.29. 1. Medicalexaminationsareconductingwith yearlyfrequency.2. The personsinvolved inBMW werevaccinatedwithHepatitis B &Tetanus DetailsProposed PlanIf Yes, provide a will be sending thelist of hazardous hazardous wastes to PCBwaste produced authorized agency.in the facility:List of hazardouswaste generatedat the granteesubmitted to thePCBIf No, provide alist ofallwasteproduced in thefacility.Page 5 of 10beingRecordsare Daily records will beMaintainedandmaintained.verifiedbytheCorporate EHSBiomedicalwaste Annual submissions willreportswillbebe done.submitted to SPCBAre annual reports on BWM submitted to SPCB as per required form (see Bio-MedicalWaste Rules 2016)?Hazardous Waste (HW)Yes NoArea of RiskIs there generation of hazardouswaste (as per Hazardous WasteRules, 2016) in the grantee?Reportsaremaintained.
Environmental Health Risk Management Plan (EHRMP)Aurobindo Pharma Limited30.Is there trained staff in the facility to identify and handle hazardous waste?31.Does the grantee have authorization fromSPCB for hazardous waste?32.Is there a secure location for storage of HW with proper signage?Are hazardous waste stored for more than 90days in the grantee’s premises?33. Is the hazardous being send to anauthorized disposal facility or user?Is the disposal facility in house?Is the disposal facility external/outsourced?34.Is a register maintained on production andtreatment, and a manifest system followedfor transport of hazardous waste from thegrantee to treatment facility?E-Waste and Batteries YesArea of Risk35.Does the grantee generate e-waste,produce or manufacture electrical andelectronic equipment?36Has the grantee obtained SPCB authorizationon e-waste? Trained staff from Trainings conducted andall departments.their records will bemaintained.AuthorizationTimely renewals andgranted from PCB. compliance of PCB rulesand regulations will bedone.Describehow Well-ventilated area andeach item is separated room will bestored –used for storing the1. platforms: are Hazardous waste duringdesigned with 2ft the Project.height from theground level ,distances fromcriticalinstallations/movementareas,dykes are usingasspillcollectors,Storedindesignatedhazardous wastestorage area.NameandTimelyrenewalofaddressof contract with the PCBfacility:Authorized third partyHWMP,will be done for thisDundigal,purpose.Hyderabad.Online manifestsRegisterswillbearefollowingmaintained throughout theaccording to PCB Project.guidelines.No DetailsProposed Plan Till now no e-waste In future, planning forgenerated.generated e-waste will besent to PCB authorized 3rdparty for recycling. NoewasteIf generated will be sent togenerated nowauthorized vendor.Page 6 of 10
Environmental Health Risk Management Plan (EHRMP)Aurobindo Pharma Limited37. Does the grantee channelize the e-waste toauthorized recycling or disposal facility?38.Does the manufacturing grantee haveExtended Producer Responsibility systemand EPR-authorization in place?39.43.Does the grantee practice reduction in the usageof hazardous substances in the manufacture ofelectrical and electronic equipment and itsparts?Does the grantee provide detailedinformation on the constituents of theequipment and their components/spares anddeclaration of conformation to Reduction inHazardous Substances in the product userdocumentation?Does the grantee maintain a record ofcollection, storage, sale and transportof e-waste? Does the grantee submit annual reports one-waste to SPCB?Is there accident reporting and records in place? 44.Are PPEs available to staff?45.Is the grantee involved in manufacture ofbatteries?46.Does the grantee generate battery waste?40.41.42. Nameaddressdisposalfacility/recycler:andofIf generated will be sent toPCB authorized vendorInhouseoroutsourcedFacility: Describe:The Will be sent to PCBgrantee is not authorized recyclermanufacturing anyelectronicequipments / theelectronicandelectric waste willbe sent to PCBauthorizedrecycler NoewasteIf generated will be sent togenerated nowauthorized vendor NoewasteIf generated will be sent togenerated nowauthorized vendor NoewasteIf generated will be sent togenerated nowauthorized vendorIf Generated will be sentto authorized recyclerInjuries ,accidents Maintaining records atOHCPPE are available The stock status of PPEwillberegularlymonitoredandprocurement will be donein time to avoid anysituation of stock out. Wearenot We are not manufacturingmanufacturing anyany batteries.batteriesMinimal quantity is Battery waste will be sentbeing generatedto authorized RecyclerPage 7 of 10
Environmental Health Risk Management Plan (EHRMP)Aurobindo Pharma Limited47.Does the grantee deposit the battery waste to er/collection center?48.In case of manufacturing, does the granteecomply toBattery Management Rules 2000 and ensurecollection of old batteries?Community Health and Safety and risk mitigationYes No Details49.50.Nameand Battery waste will be sentaddressof to authorized drecycler No manufacturingWe are not manufacturingof batteriesany batteriesSafety Transportation Management System (for transportOf hazardous material)Emergency preparedness and participation of local authorities and potentially affectedcommunitiesProposed PlanTrem cardsSafely Transported byPCB authorized agencyIncidentmanagementOn site emergency PlanmaintainedOtherArea of Risk51.52.5354.55.Yes No DetailsDoes the grantee use any radioactivematerials (isotopes tracers, radiationequipment, etc)?Does the grantee have appropriateradioactive material and waste storageand disposal system in place?Are radioactive warning signs inplace? Is the lab/room air regularlycheckedformicrobialcontamination?Are there any odor control measures in place? Are fume hoods and exhaustsregularlycheckedandmaintained?Does the grantee use DG set 15 KVA?Does the grantee have consent for DG 15 KVA? Are emissions from boilersand DG sets regularlyProposed Plan No istopes / radiation In future also will notequipmentuse Describe: No istopes / In future also will notradiation equipment use No isotopes / radiationIn future also will notequipmentuseEnvironmentalCleaning regularlymonitoring and AHU’SCleaningCleaning and maintainrecordsThepreventive Fume cup boards aremaintenance is in place periodicalcheckingfor the fume cup boards with PM SOPsGrant form PCB and Control air emissionspower departmentfrom stack monitoringby third party and willmaintainthesecontrolling measuresthroughout the Project.Page 8 of 10
Environmental Health Risk Management Plan (EHRMP)Aurobindo Pharma Limited56.57.58.monitored to be within theprescribed norms?Does the grantee have properdisposal process for solid andplastic waste in compliance toSolid Waste Management Rules,2016andPlasticWasteManagement Rules, 2016?Iswastewatertreatedseparately by the grantee?(Liquidwastefromlaboratory, chemicals, fluids,solvents,mediumandcultures, coolants, etc.) Describe: solid wasteSolidwastearecarboys,plasticsegregated and sent tocontainersPCBAuthorizedVendor. Types of wastewater: Periodic checks willTreatmentofwastewater: Kill tankAre there any settlements, waterbodies, cultivated land, or any othereco-sensitive areas near thegrantee’s premises?60.Are there any buffers, fire vehicle routes in the grantee’s premises?sent to CETP.Chemicalmanagementinwastewater treatmentplants: No sludge from process If generated will besegregatedanddisposed as per PCBDescribe : Boiler area Utilization ear budsby using PPE/ear muffs during theproject to mitigate thisrisk in future. Describe:andNo Locatedcultivation land near by surroundedbyindustrial areaDistancefrompremises:Roads on all the sides are Fire vehicles will beeasy access for fire accessedinourvehicles.existing premisesAre there sludge management andcut off drains in place forwastewater?Are necessary provisions for noise cancellation in place?59.be done and thetreated water will beCOVID Precautions & Guidelines Implementation61.Guidelines of CPCB/SPCB/GoI for Handling, Treatment, and Disposal ofCOVID Waste Generated is whetherbeing followed?62.SOP on preventive measures to contain spread of COVID-19 issuedby ICMR/GoI from time to time iswhether being followed?Treatment will be done Afterautoclavingby autoclaving 121 and waste will be disposed.degree per hour and thespillages are handlingwith treating of 10%sodium hypochlorite.Handling of spillages in ImplementationofViral Vaccine Pilot plant instructionsasPRD/SOP/130.00mentioned in SOPs toavoid the spillages ofacid,alkali,flammable, chemical,biologicalandPage 9 of 10
Environmental Health Risk Management Plan (EHRMP)Aurobindo Pharma Limitedrecombinant materialswill be ensured.Notwithstanding the above other risk (relevant to the project activities) that will be identifiedin the course shall be addressed as per standard mitigation monitoring parameters and mannerof records keeping shall be in accordance to the recommendations of the project monitoringcommittee on subject experts engaged by BIRAC.Page 10 of 10
biomedical waste produced in the facility: Decontaminated cell stacks, Media Plates If No, provide a list of all waste produced in the facility. Biomedical waste will be sent to PCB authorized third party. 15. Is there trained staff to handle biomedical waste in the grantee? Staff trained on the decontamination process