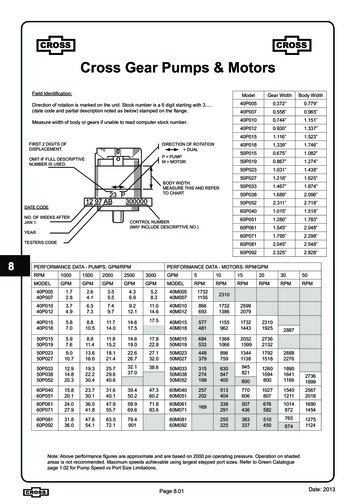
Transcription
Global Procurement & Materials (GPM)Keysight Technologies
1. Objectives & Scope2. Eight Discipline (8D) Approach Guideline3. Intelex System GuidelineKeysight Restricted2
Keysight Restricted3
This document guide suppliers To identify the real root cause and implement the corrective action effectively to prevent the recurrence. To answer the Supplier Corrective Action Request (SCAR)/External Sub Case (ESC) through IntelexSystem. To cascade the Keysight requirement to suppliers Supplier shall respond to Supplier Corrective Action Request (SCAR) within fourteen days uponsupplier receipt of the defective part. Supplier may extend the supplier’s response time to morethan fourteen days with valid justification. All Keysight SuppliersKeysight Restricted4
8 D AP P R O AC tionD4Root sD6RiskManagementD5CorrectiveActionKeysight Restricted5
Establish a small group of people with the process or product knowledge, allocate time, authority, and skill in the required technical disciplinesto solve the problem and implement corrective actions.Guideline:Team members should include as below (but not limited to) Cross Functional or Multi Disciplinary Process Owner & Technical Expert Others involved in the containment, root cause analysis, correction and prevention of theExample:NameDesignationEmail AddressAshly Tan CYProduct Engineerashly Tan@supplier.comJackson BillQuality Engineerjackson bill@supplier.comYeong Wei TingProcess Engineerweiting yeong@supplier.comKeysight Restricted6
Problem verification is the first step of problem investigation. There are 3 main activities: Verify the problem Collect information Describe the problemGuideline:To describe the problem specifically, (5W2H) terms (who, what, where, when, why, how, and how many) would help.5W2HQuestions to askAnswer should be providedWhoWho first observed? / Who is affected?- Location of defect foundWhatWhat type of problem? / What has the problem? / What is happening?- Failure reported/ Part Number/ Model/ Detail description of failureWhyWhy it is a problem?WhereWhere was the problem observed/ occur?WhenWhen the problem first noticed?How much/manyHow often- Detail description on the failure and verification done/ Date code ofdefective partHow much/ many involved?- Quantity affectedWhat is the trend? Has the problem occurred previously?- Failure historyExample: SCAR Number: 1234-SCA-567-ESC-890 Keysight Part Number: 1234-5678 Failure Description: 1234-5566 is received instead of the correct part (1234-5678). One piece rejected out of total received quantityhundred pieces. The defective part date code is 0421. This is the first case reported from customer.Keysight Restricted7
Containment action is to limit a problem extent while continue normal operation until the root cause is defined and permanent corrective actionis implementedThe containment area should cover production, finished goods, customer on hand (Keysight), Incoming material, and Warehouse Storage.GuidelineContainment action Stoppage of production or shipment Segregation goods on pass or fail Additional visual control Informing customer about the problem Informing operators about the problem Check on similar product or processes if there is similar riskExample:No.Containment Action PlanInventory LocationImplementation DateResponsible PersonResults1100% screeningSupplier Production30/04/2021Supplier0 pcs/1000 pcs rejection(Date Code 0321 and 0421)2100% screeningSupplier Warehouse30/04/2021Supplier0 pcs rejectionKeysight Restricted8
Root cause identification is the most important step. The problem will be solved only if the corrective action implemented is addressing the realroot cause accurately.Root Cause Analysis (RCA) is a systematic approach to identify the actual root causes of a problem.GuidelineBelow are the tools frequently used in RCA. 5 Whys Analysis OR Fishbone Diagram (Cause and Effect Diagram)Notes: The RCA should identify root cause for both Occurrence (Why it occur?) AND Detection (Why it can’t be detected?)Keysight Restricted9
5 W H Y S A N A LY S I S5 Whys Analysis (or Why-Why Analysis) is a continuous question-asking technique used to explore the cause-and-effect relationshipsunderlying a particular problem.Guidelinei.Define the problem.ii.Ask Why the problem happen and write down the answeriii.Validate the answer is it the real root causeiv.If no, Repeat step 3 until problem’s root cause is identified.Keysight Restricted10
Evaluate the effectiveness ofcorrective action to identify thereal root cause5 W H Y S A N A LY S I SExample (Occurrence):WhyQuestionsAnswerCorrection ActionWhy 1Why the wrong part shipped to Keysight?Wrong part was pull from the inventoryRetrain the stock picker- almost no benefit.The cause is come from mislabel from supplierWhy 2Why was the wrong part pull from the inventory?The part was mislabeledPerform inspection on inventory- minimum benefit Apply to onhand inventory onlyWhy 3Why was the part mislabeled?Our supplier mislabeled the part before ship to thewarehouse.Contain the problem by sorting out the mislabel part -very limitedlong-term benefitWhy 4Why your supplier mislabeled the part before shipto the warehouse?The operator at supplier site took the other label andplaced at the productConduct training for operator – limited long-term benefitWhy 5Why the operator at supplier site took the otherlabel and placed at the product?Many labels with different order was printed at thesame location everyday so it is easy to causemislabeledRevise the process flow, only print one order at one location. Clearthe location before proceeding to the subsequent order. (Mistakeproof printing label process or application) – highly effectiveEvidence & Summarye.g., Supplier FailureAnalysis Report, processflow etc.Example (Escapee):WhyWhy 1QuestionsWhy was the wrong part not detected?Answera)b)Why 2a)b)Why was the operator miss out to detectthis?Why was the in-process inspection do notinspect the label?a)b)Correction ActionOutgoing Inspection (OQA) Operator miss out todetect thisThe in-process inspector do not inspect the label.a)b)Retrain the operator – almost no benefit.Alert the operator to check the label– almost no benefit.The operator just check the label withoutchecking the physical part.This is not stated as checking criteria in the WorkInstruction.a)Create alert notice for OQA operator to create awareness .limited long-term benefitEnhance the in-process inspection criteria to includeinspection of the label versus physical part. (To detectmislabeled parts at the earlier stage) - highly effectiveKeysight Restrictedb)Evidence & Summary11
F I S H BO NE D I AG R AM ( C AU S E AN D E F F ECT D I AG R AM )A fishbone diagram is a visualization tool for categorizing the potential causes of a problem in order to identify its root causes.Guidelinei.Define the problemii.Identify the key causesiii.Brainstorm the causesiv.Validate the identified root cause causes.Keysight Restricted12
F I S H BO NE D I AG R AM ( C AU S E AN D E F F ECT D I AG R AM )Example:1.Identify potential root causeCauseWrong part pullfrom inventoryTools wear andtearNomaintenanceMETHODMANMACHINENo inspectionNo propercommunicationNo trainingNot capableLack ofunderstandingNo agreementwith supplierNot approvedby vendorMATERIALEffectLack ofawarenessWrong labelon the productNot listed incriteriaProblemDescription:Wrong Part sent tocustomerWrong LLMfrom supplierMessyproduction flowNo IQAInspectionENVIRONMENTKeysight Restricted13
F I S H BO NE D I AG R AM ( C AU S E AN D E F F ECT D I AG R AM )Example:2.Validate identified root causeCategoryPotential Root causeValidation / investigationsFindingsConclusion: True / falseManWrong product pull frominventoryThe operator pull the product by partnumber following the process.This is not the root causeFALSEManLack of awarenessRetraining is conducted half yearly.This is not the root causeFALSEMethodWrong label on the productThe label is the correct part number (12345678), but the physical part is 1234-5566.Many labels with differentorder was printed at thesame location everyday so itis easy to cause mislabeled.TRUEMaterialWrong LLM from supplier . .Keysight Restricted14
Corrective action (CA) is to remove the root cause and prevent a problem from ever happening again.The corrective action should correspond to the root cause identified earlier in order to eliminate the real root cause and prevent recurrenceof the problem.Method such as brainstorming is recommended as it can help to select appropriate corrective action for identified root cause.GuidelineFor root cause of “Why problem occur?”For root cause of “Why not detected?” Introduce additional process control Introduce new process Introduce new testing gate Enhance previous testing coverageExample:ResponsiblePersonImplementation DateRevise the process flow, only print one order at onelocation. Clear the location before proceeding to thesubsequent order.Supplier15/05/2021Closee.g., new process flowUpdate in-process inspection work instruction toinclude criteria to inspect the label versus physicalpart.Supplier30/05/2021Closee.g., new inspectionwork instructionArea of FocusCorrective ActionOccurrenceDetectionKeysight RestrictedStatusEvidence15
An action to eliminate the causes of nonconformities in order to prevent recurrence which is permanent and prevent any similar cases tooccur. These actions are proactive and oriented towards a potential event in the future.Preventive action need to be taken if there is any identified risk.Guideline Update or modify management processes Implement the corrective action to Keysight product with similar design or product using the same process. Update controls system Failure Mode & Effect Analysis (FMEA), Process Control Plan (PCP), work instructions, quality alerts andprocedures Changing the process parameter/ product specificationNote: Preventive action shall not be the same as Corrective ActionExample:Area of reventive ActionImplementationDateStatusUpdate the control in FMEA document andprocess control plan.Supplier QA15/05/2021CloseImplement corrective action in D5 to similar parts(KPN 1234-5566 and 8765-4321)Supplier30/05/2021CloseKeysight RestrictedEvidenceUpdated FMEAand PCP.16
After the corrective actions is implemented, the effectiveness should be verified.The key to verification is evidence. This evidence usually takes the form of data, records or first-hand observations.It is recommended the verification made by monitoring the quality of next deliveriesGuidelineItems to be included (but not limited to the following) Monitoring Method (e.g., Type of testing, monitoring area and expected results) Duration of monitoring (minimum three months) Serial number/Batch/ Lot Number (minimum three batch/lot should be inspected) Evidence of monitoring (e.g., testing data, inspection results)Note: For low volume production, batch/lot required could be reduced (with justification and consensus from Keysight)Example:Monitoring MetricsMonitor the next three lots for anyrecurrenceResponsiblePersonSupplierMonitoring PeriodMonitor for threemonthsKeysight RestrictedResultsMonitored for three lots, date code200920, 201003 and 201117. Nosimilar issue reported.Evidence*Attached theoutgoing inspectionresults17
To congratulate and recognize the team efforts and special team member contribution.This is a good section to document lessons learned.Example:NameDesignationEmail AddressAshly Tan CYProduct Engineerashly Tan@supplier.comJackson BillQuality Engineerjackson bill@supplier.comYeong Wei TingProcess Engineerweiting yeong@supplier.comKeysight Restricted18
TermDefinition8DEight DisciplineRCCARoot Cause and Corrective ActionSCARSupplier Corrective Action RequestFMEAFailure Mode and Effects AnalysisPCPProcess Control PlanCACorrective ActionKeysight Restricted19
Keysight Restricted20
Step 1:Log in to IntelexSystemStep 2:Acknowledgethe ESCStep 3:Submit the 8DReportKeysight RestrictedStep 4:Verify theCorrectiveActionEffectiveness21
Email NotificationSteps1 ESC Owner SCA Owner ESC Owner 1.The following email will be sent to the Supplier (ESCOwner), copying the SCA Owner and other recipientsin the Distribution List.2.Click the Link “ here ” in email to access to theassigned ESC.3.Log in to Intelex using the username and password set23Keysight Restricted22
Now you are hereESCKeysight Restricted23
Steps31.Review information provided in “InitiationDetails” session.2.Click “ Edit ” to fill in acknowledgementMessage and Part/Info Arrival Date.3.Click “ Acknowledge “to complete task1AD Link2Keysight Restricted24
Email Notification SCA Owner ESC Owner ESC Approver ESC Owner Supplier SCA Owner Keysight Restricted25
Now you are hereKeysight Restricted26
Steps312Keysight Restricted1.After complete 8D Review with Keysight SubCase Owner and Master Case Owner, submitESC byclicking “ Attach Documents ” to attach 8Dreport, related files and meeting minutes2.Provide a brief summary of Root Cause,Corrective Action and Verification Actiondone in “Response Summary”.3.Click “ Save ” then click “ Submit ” oncecompleted.27
Email Notification SCA Owner ESC Owner ESC Approver ESC Owner SCA Owner SCA Owner Keysight Restricted28
Now you are hereKeysight Restricted29
Steps ESC Owner 1 SCA Owner 1.The following email will be sent to the Supplier (ESCOwner), copying the SCA Owner and otherrecipients in the Distribution List.2.Once the monitoring is completed, click “ here ” toassess the ESC ESC Owner Keysight Restricted30
Steps31.Click “ Edit ” at the top of the page & Click “ AttachDocuments ” to attach verification data.2.Under Effectiveness Details, select “Yes” or “No” and providesummary of CA effectiveness verification.3.Click “ Complete ” at the top of page. The process iscomplete.12Keysight Restricted31
Keysight Restricted32
Root cause identification is the most important step. The problem will be solved only if the corrective action implemented is addressing the real root cause accurately. Root Cause Analysis (RCA) is a systematic approach to identify the actual root causes of a problem. Guideline Below are the tools frequently used in RCA. 5 Whys Analysis OR