Transcription
2020 IJRAR March 2020, Volume 7, Issue 1www.ijrar.org (E-ISSN 2348-1269, P- ISSN 2349-5138)PASSIVATION:TO QUALIFY EQUIPMENT FORINSTALLATION QUALIFICATIONManjot Singh Narula, Graduated from Chandigarh University1. ABSTRACT:Passivation is a non-electrolytic process by using nitric or citric acid which removes free iron from the surface and forms an inert, protective oxidelayer that in turn renders the stainless steel more rust-resistance because of shortage of iron on surface to react with the atmosphere.Passivation of stainless steel is to clean its surface using an acid to remove free iron and to create a chromium rich surface. The most generalstainless steel alloys used to manufacture pharmaceutical equipment are types 316L, 316, 304, 304L. However above alloys are generally iron.In the 300 series stainless steel, the corrosion resistance is given by a molecular layer called passive layer. The passive layer does not have the samecomposition as the bulk alloy. It has mostly iron and chromium in the form of chromium oxide. So, after following proper methodology ofperforming passivation, Standard results are compared with the obtained results to observe the difference in both the results.CHEMICAL COMPOSITION % 0800.0308.0-12.00.080NOTE: If the passive layer has more iron, is more prone to corrosion. If the passive layer has maximum chrome, is more corrosion resistantKeywords: Corrosion, Installation qualification.2. OBJECTIVES OF RESEARCH : To evaluate equipment for its resistance capability to corrosion. To identify the capability of equipment for processing of manufacturing of dosage form. To determine corrosive capability of any equipment.3. LITERATURE:Passivation of stainless steel is done to clean its surface using an acid for the purpose to remove free iron and create achromium rich surface.IJRAR2001826International Journal of Research and Analytical Reviews (IJRAR) www.ijrar.org822
2020 IJRAR March 2020, Volume 7, Issue 1www.ijrar.org (E-ISSN 2348-1269, P- ISSN 2349-5138)3.1 Regulations:21CFR211.65(a)“Equipment shall be constructed so that surfaces that contact components, in-process materials, or drug products shall notbe reactive, additive, or absorptive so as to alter the safety, identity, strength, quality, or purity of the drug product beyondthe official or other established requirements.”In the Food and Drugs Act requires:“No person shall sell any drug that Was manufactured, prepared, preserved packaged, or stored under unsanitaryconditions”Good Manufacturing Practices (GMP) Guidelines - 2009 Edition, Version 2 (GUI-0001)“C.02.005 The equipment with which a lot or batch of a drug is fabricated, packaged/labelled or tested shall be designed,constructed, maintained, operated, and arranged in a manner that prevents the contamination of the drug and the additionof extraneous material to the drug;”Some substances common in the drug manufacturing process are corrosive even to the 316L alloy. Those substances are: High purity water (purified water, WFI etc.) Pure steam Saline solutions Chlorine compounds (Benzalkonium chloride, hydrochloric acid, etc.)To improve the chemical resistance of stainless steel then this must be passivated. It is a common industrial practice thatall stainless steel surfaces in contact with raw materials or finish product should be passivated to prevent any potentialreaction of raw material with the alloy.3.2 CHEMICALS IN USE:The two most common chemistry to do the process of passivation stainless steel in the pharmaceutical industry are:1) Nitric acid passivation2) Citric acid passivationNitric acid was the first acid to be use to passivate stainless steel.Advantages: Low cost. It can be use cold Require less contact time than citric acid. Same nitric acid solution can be used multiple times.Disadvantages: It can be hazardous Additional to dissolve iron, it also dissolves heavy metals that are toxic It must be disposed off as it will not keep the metals dissolved after neutralizationCitric acid is very common used to passivate stainless steel in the pharmaceutical industry, and it has the followingAdvantages: It comes with Food grade and USP grade It is not hazardous It dissolves only iron, not the heavy metals that enrich the alloys. Its performance can be enhanced using other agonist chemicals to generate passive layers better than nitric acid . It will keep the iron dissolved after neutralization. It is biodegradableDisadvantages: It is more expensive than nitric acid Low concentrations solutions of citric acid will require to be heated up to 180⁰F (80 ⁰C)IJRAR2001826International Journal of Research and Analytical Reviews (IJRAR) www.ijrar.org823
2020 IJRAR March 2020, Volume 7, Issue 1www.ijrar.org (E-ISSN 2348-1269, P- ISSN 2349-5138)3.3 PASSIVATION APPROACHES: Recirculation: It is very useful way to clean pipes and internal surfaces of equipment designed to hold liquids.Immersion: This method is good for parts which can be dip in chemical tanks.Spray: This approach is good for tanks, or other parts, reduces the amount of chemical required.Foam: It is useful for surface which cannot be cleaned by recirculation or immersion, when foam method is usedthe liquid remains in contact with the surface for long time than sprayed solution. Gel or pastes: It is useful method for surfaces that can be reach by hand.3.4 VERIFICATION FOR EFFECTIVE PASSIVATION:The effectiveness of applied passivation procedure must be evaluated to confirm as to whether the surface contamination orfree iron has been removed and an inert protective or chromium rich layer is formed on the stainless steel surface. Varioustests are conducted to determine the quality of passivation, which includes water Immersion Test, High Humidity test,Copper Sulfate Test etc. Other tests like XPS (X-ray Photoelectron Spectroscopy) which is used for analysis of oxidationstates of element while AES (Auger Electron Spectroscopy) assess the chrome/iron ratio on the metal surface. Also afterpassivation, more tests can be performed to verify whether passivation has been properly performed. This includesexposure to a salt-spray for up to two hours, or high humidity conditions for atleast twenty-four hours. A commonly usedmethod of verification is to immerse the passivated part of stainless steel in a copper sulphate solution (CuSO4) for a periodof six minutes and then rinse and examine the immersed part. If there is any pink color visible, it indicates the presence offree iron. This means the passivation process was not satisfactory.3.5 Case Study 1: Environmentally-Preferable Alternative to Nitric Acid PassivationBenefits Replacement of nitric acid passivation with citric acid reduces environmental, safety, and health risks, and materialhandling and disposal costs Leveraged resources from other agencies reduced the cost of testing for NASAChallengeThe National Aeronautics and Space Administration(NASA), the Department of Defense (DoD) and the European SpaceAgency (ESA) have similar equipment and processes that require the passivation of stainless steel for protection againstcorrosion. Passivation forms ametal oxide layer that reduces the impact of environmental factors. The standard practice has been to use nitric acid forpassivation of stainless steel alloys. While nitric acid exhibits excellent performance, there are numerous environmental,safety, and operational issues associated with its use. Nitric acid used to passivate stainless steel results in a large amount ofhazardous waste and nitrogen oxide emissions, and the process includes use of hazardous materials. Nitric acid passivationrequires a 25% or 50% concentration of the strong acid.SolutionCitric acid is an alternative to nitric acid for the passivation of stainless steel. Citric acid offers various benefits includingincreased safety for personnel, reduced environmental impact, and reduced operational cost. While citric acid use hadbecome more prominent in industry, there was little evidence that citric acid is a technically sound passivation agent,especially for the unique and critical applications encountered by NASA and ESA. ITB worked with NASA centers(Kennedy Space Center (KSC), Stennis Space Center, Wallops Flight Facility, the NASA Corrosion TechnologyLaboratory, the NASA Ground Systems Development Program, DoD, and ESA to evaluate citric acid as a safer alternativeto nitric acid for stainless steel alloys passivation. Testing results have shown that citric acid performs as well as, or betterthan, nitric acid regarding corrosion resistance. The citric acid passivation also does not affect adhesion of subsequentlyapplied coatings. Overall, regardless of alloy, the higher citric acid concentration, temperature, and bath dwell time yieldedthe best results. As the citric acid concentration increased, the percent of panels that passed also increased.Use of citric acid reduces the environmental, safety, health risks, and material handling and disposal costs associated withthe use of nitric acid. Citric acid removes free iron from the surface while leaving behind beneficial heavy metalspresenting operational benefits. Citric acid immersion baths also retain their potency longer, thus requiring less frequentrefillingand reduced volume and potential toxicity of effluent and rinse water.IJRAR2001826International Journal of Research and Analytical Reviews (IJRAR) www.ijrar.org824
2020 IJRAR March 2020, Volume 7, Issue 1www.ijrar.org (E-ISSN 2348-1269, P- ISSN 2349-5138)ITB ApproachA fundamental activity of ITB was the testing and validation of technologies that could potentially reduce environmentallydriven risks to NASA’s mission. Testing of such materials or processes is necessary to obtain data on their performanceprior to implementation. ITB employs the unique approach of distributing costs among project participants. ITB reducesNASA’s cost burden by creating partnerships that provide additional funding, resources and expertise for its projects. Thesecollaborations reduce the individual contributors’ shares of total research costs and the duplication of effort that mightotherwise occur if individual organizations worked alone.3.6 Case Study 2: Corrosion Investigation of Pharmaceutical Clean Steam SystemsOverview:Pharmaceutical clean (pure) steam systems consist of a generator,distribution tubing or piping, thermodynamic or balancedpressure thermostatic traps, control valves, pressure-reducing regulators, pressure gauges, pressure-relief valves, andvolumetric totalizers. Most of these components are made of 316L stainless steel and contain fluoropolymer gaskets(mostcommonly polytetrafluoroethylene, also known as PTFE or Teflon), as well as semi metallic or other elastomeric materials.These components tend to corrode or degrade in service, potentially compromising the quality of the final clean steam (CS)utility product.This project investigated stainless steel coupon samples from four CS system case studies, testing condensate for metalsand particles, and conducting a risk assessment of potential corrosion effects on process and critical utility systems.Examining the corrosion byproducts involved preparing sample coupons of corroded tubing and components fromDistribution systems. These case studies investigated a variety of surface conditions, and included analysis of typical rougeproducts and corrosion effects. The referenced sample surfaces were evaluated for rouge deposits by visual inspection,scanning electron microscopy (SEM), auger electron spectroscopy (AES), and electron spectroscopy for chemical analysis(ESCA)/ x-ray photoelectron spectroscopy (XPS). These techniques reveal the physical and atomic properties of thecorrosion and deposits, and identify potential contributions to the critical utility fluid properties or final product.Summary:From system inception, when a new CS generator and distribution tubing is first commissioned and energized, severalpotential factors for corrosion are present: In addition to clean steam, the CS generator begins to generate corrosion particles (class 1 rouge) that have thepotential to migrate. Separately, pressure regulators begin to generate (class 3) rouge downstream, and possibly upstream as a functionof time. High levels of delta ferrite, metallic inclusions, or other material defect content in components begin to generatecorrosion products (class 2 rouge). Condensate traps can add further migration-capable corrosion (class 1 rouge). Distribution tubing will show corrosion effects and accumulated rouge (class 2 and 3 rouge). Ball valves can generate corrosion from trap lines as well as at use points.Further, as a function of time, these corrosion factors may produce corrosion products as they meet, combine, and overlapwith a blend of ferrous and ferric rouge. Generally, black rouge is first seen in the generator; rouge then emerges at thegenerator discharge piping and eventually throughout the CS distribution system.Conclusion:The corrosion byproducts encountered in clean and pure steam systems carbon, silica, and iron oxide compounds arepresent to some degree in every system. Many CS systems lack proper routine inspection and maintenance that couldcontrol corrosion and particulate migration from oxide deposit exfoliation. Corrosion problems are exacerbated by poorgasketspecifications, components with dissimilar metals, and decreased stainless steel surface quality, as well as the uncontrollednature of mechanical/electrochemical polishing materials and methods combined with poor material handling and lack ofroutine derouging and passivation techniques. It has also long been suspected that stainless steel materials are notnecessarily delivered at a desirable quality level. Manufacturing processes, combined with subsequent material handlingunit operations and fabrication techniques, establish the surface chemistry (chromium oxide content of the passive film),corrosion resistance, and surface finish quality, which all affect the final product. Claims that these grayish/black depositsare stable, inevitable, and should be left alone have very little credibility. Corrosion produces rouge that is evidenced asdiscolored stains on product contact surfaces, and generates mobile particles that accumulate on steam sterilized surfaces.CS rouge contaminants have been found in final filtration processes, becoming a potentially uncontrolled material in thefinal process fluids and gasses. While we acknowledge that the examples presented here are specific cases, they are notunique. They are similar to cases found within other systems,IJRAR2001826International Journal of Research and Analytical Reviews (IJRAR) www.ijrar.org825
2020 IJRAR March 2020, Volume 7, Issue 1www.ijrar.org (E-ISSN 2348-1269, P- ISSN 2349-5138)especially those where corrosion has been left to continue without proper corrective treatment. Finally, corrosion within CSsystems will generate migratory rouge that canbe identified and measured in the steam, the condensate, and on the system interior surfaces. Proper design andmaintenance is critical in the operation of CS generation and distribution systems for high-purity applications. Futureresearch measuring the time, conditions, and properties of rouge development could be compared to particulate generationto establish riskof product contamination. Systematic, routine measurements over a two year period could track corrosion products toillustrate changes in particulate release and transport within the CS system studied, as well as the surface conditions withinthe generation and distribution equipment.3.7 QUALIFICATIONS:Before you conduct validation, you must complete the process of qualification. It is a systematic process that starts by thephases of the installations, equipment and utilities. Systems and equipment should be appropriately designed, located,installed, operated, and maintained to suit their intended purpose. Analytical Instrumentation Qualification, also known asAIQ, is the documented process where a complex and sophisticated measurement device is demonstrated to be accurate,precise and selective enough for the intended analytical measurement. This is carried out to determine the sustainability andqualification of any instrument for the intended purpose qualification is not limited to a validation process, but it is a part ofit. It can be further divided into design qualification (DQ), installation qualification (IQ), operation qualification (OQ) orperformance qualification (PQ). Based on the operation and function of equipment, system or utility, you must makeinstallation qualification and operation necessary. They should be monitored and calibrated periodically and they must besubmitted to preventive maintenance. New systems and equipment should pass through all stages of qualification including Design qualification (DQ), Installation qualification (IQ), Operational qualification (OQ) Performance qualification (PQ) as mentioned in the following lificationIJRAR2001826International Journal of Research and Analytical Reviews (IJRAR) www.ijrar.org826
2020 IJRAR March 2020, Volume 7, Issue 1www.ijrar.org (E-ISSN 2348-1269, P- ISSN 2349-5138)Design Qualification (DQ) of Equipment User requirements should be considered when deciding on the specific design of a system or equipment.A suitable supplier should be selected for the appropriate system or equipment.Installation Qualification (IQ): It evaluates means of accomodating new equipment and testing its materials.Systems and equipments should be correctly installed in accordance with an installation plan and installationqualification protocol. Installation qualification should include identification and verification of all systemelements, parts, services and controls. There should be documented records for the installation which shouldinclude the details of supplier and manufacturer, system or equipment name, model and serial number, date ofinstallation, spare parts, relevant procedures and certificates. For installation qualification, we’ll first look at theequipment material. For example, if we specified 316 stainless, we’ll test to verify it is in fact 316 stainless.Sometimes stainless steel is passivated and you can test to verify there are no further residues from the passivati onprocess.You might have specified a 5 force power motor in your equipment. For example, You want to check toensure it’s a 5 force power motor. You’ll also confirm that the power output and the power requirements areconsistent with your specifications, and the room that the equipment is installed in can accommodate that powersource. Once you have completed your review of the installation and everything is in order, you can trust that theequipment is going to operate the way in which it was designed.Operational Qualification (OQ): It is Essential to Challenge Your Equipment Parameters. The next phase isOQ, operational qualification. Systems and equipment should operate correctly and their operation should beverified in accordance with an operational qualification protocol. Operational qualification should includeverification of all system elements, parts, services, controls, gauges and other components. Standard OperatingProcedures (SOP) for the operation should be finalized and approved. At this stage, if you’ve specified that yourequipment is going to runs at reccomended RPM according to standard protocol i.e. batch manufacturing record(BMR) and will draw a specific amount of power, you want to verify that the equipment is achieving thoseoperational requirements. So, review those parameters and challenge them. Again, make sure your equipmentactually runs the way it’s supposed to run.Performance Qualification (PQ): It puts your equipment to the final test. Systems and equipment shouldconsistently perform in accordance with design specifications. The performance should be verified in accordancewith a performance qualification protocol. There should be documented records for the verification of performanceto indicate the satisfactory performance over a period of time. Manufacturers should justify the selected period overwhich performance qualification is done. In the PQ (performance qualification), we like to challenge theequipment, but now under load. While it runs at recommended RPM according to standard protocol i.e. batchmanufacturing record (BMR) when it’s empty, what happens when there’s 300 kilos of material in it? Can it stillachieve those speed ranges? That’s the essence and focus of the PQ phase. Once you’ve completed these threephases, the equipment is available for use in whatever process you intended for it.What are Ideal Characteristics of an Outsourcing Partner for Qualification?The ideal outsourcing partner for Validation, Qualification and Calibration services should: Adopt a strong Quality Management SystemProvide a staff of qualified and trained technicians and engineersPossess an adequate number of equipment, managed and routinely checked as required by GMPAssure high quality standards, both in technical competencies and in documentationDemonstrate a successful track record and financial stability.IJRAR2001826International Journal of Research and Analytical Reviews (IJRAR) www.ijrar.org827
2020 IJRAR March 2020, Volume 7, Issue 1www.ijrar.org (E-ISSN 2348-1269, P- ISSN 2349-5138)4. LITERATURE REVIEW:1. Benvenuti marco, “Validation, qualification and Calibration in a pharmaceutical” studied the three principles: validation, qualification and calibration. Validation is a systematic approach to collecting and analysing sufficientdata to give reasonable assurance and documented evidence that a process or an analytical method will, whenoperated within specified parameters, consistently produce results within predetermined specifications. When thisapproach is related to a machine or equipment, rather than Validation, this is referred to as Qualification.Qualification is part of, but not limited to, a validation process, which in turn can be divided into InstallationQualification (IQ), Operation Qualification (OQ), or Performance Qualification (PQ). In performing theseactivities, many documents describing plans and approaches to analysis are generated. These include ValidationMaster Plan, Qualification Master Plan, GMP Risk Analysis, Validation Protocol, Test Protocol (includingspecification), Validation Report, and finally a Summary of Deviations/Issues. Calibration is of utmost importancein building a solid Quality System Management with experts and well-trained specialists. Additional externalsupport is also requested when refurbishing, upgrading and building departments or plants and when buying newequipment or machines. In this case, service providers can also be contracted to perform and follow, on behalf ofpharmaceutical companies, Factory and Site Acceptance Tests (FAT & SAT).2. Choudhary Ankur, “Qualification of systems and equipments in pharmaceuticals”, studied the principle andscope of qualification of systems and equipment. He also studied about the design qualification of equipment,installation qualification of equipment, operational qualification of equipment and performance qualification ofsystem which are the main parameters to qualify equipment. Along with this, Requalification and qualification of “in-use” systems and equipment is also studied.3. Fuchs John, “What is Passivation”, gave the detailed study of word passivation in which the word passivation isnormally associated with stainless steel, there are several other metals that can be “passivated” in one way oranother. The root of the word passivation, of course, relates to the passivity of the metal. In simple terms, thepassivated metal is protected by a passivation layer from reaction with oxygen, water, chlorine ions and othersources of potential corrosion.4. Choudhary Sachin, “Passivation of stainless steel in pharmaceuticals”, in this author gave information aboutthe passivation basics, degreasing, passivation and how the effective passivation can be verified.5. Kessel k, 2016, Alternative to Nitric Acid Passivation, studied about the environmentally preffered alternative tonitric acid passivation which have many benefits like Replacement of nitric acid passivation with citric acid reducesenvironmental, safety, and health risks, and material handling and disposal costs and Leveraged resources fromother agencies reduced the cost of testing for NASA6. Drew C. Coleman and Daryl L. Roll,” corrosion investigation of pharmaceutical clean steam systems”,presented the research on the problem of rouge in clean steam generators and their distribution systems, as well aspossible deleterious effects on capital equipment and final drug product.5. PLAN OF WORK:1)2)3)4)Selection of Equipment/Instrument ( In this research ,Sieve is used)Procurement of Instrument to perform experimentPreparation of Acidic and basic solution for testingMethodology includes preparation of solutions using 5% w/v citric acid and 5% w/v NaOH and analysis by UVspectroscopy5) Analysis and Report of work6) Interpretation and submitting ResultIJRAR2001826International Journal of Research and Analytical Reviews (IJRAR) www.ijrar.org828
2020 IJRAR March 2020, Volume 7, Issue 1www.ijrar.org (E-ISSN 2348-1269, P- ISSN 2349-5138)6. METHODOLOGYInstrunment is selected on which passivation is to be done.Sieve was procured from Printo Pack Machines firm.5% w/v NaOH basic solution in 2000 ml water was preparedSome amount of this solution was kept aside as a reference basic solution5% w/v Citric acid solution in 2000 ml water was preparedSome amount of this solution was kept aside as a reference acidic solutionRest amount of solution was poured in chamber in which sieve was placed.Fig. 1 (a) & 1 (b)Then left undisturbed for 24 hours.Then after 24 hours, sample of solution was taken.Fig. 2(a) & 2(b)Then UV spectroscopy of samples was donePeaks of both sample and refrence solutions ( acidic & basic ) were observedPeaks were compared and result was noted downIJRAR2001826International Journal of Research and Analytical Reviews (IJRAR) www.ijrar.org829
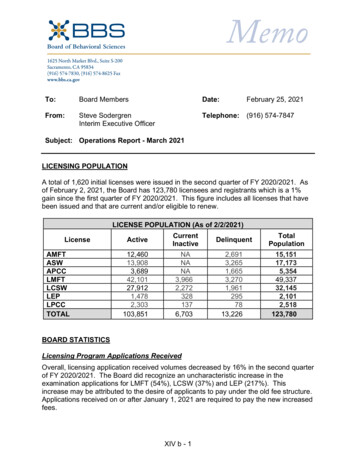
2020 IJRAR March 2020, Volume 7, Issue 1FIG. 1 (A)FIG. 2 (A) (BASE)www.ijrar.org (E-ISSN 2348-1269, P- ISSN 2349-5138)FIG. 1 (B)FIG. 2 (B) (ACID)7. RESULT:UV spectroscopy of blank (5% w/v NaOH& 5% w/v HCl) and stock (acid & sample solution containing sieve) hasperformed. Standard peak of 5% w/v NaOH and 5% w/v citric acid has been taken. Standard peak of 5% w/v NaOH isshowing as green colour on graph ( Graph (A) and Standard peak of 5% w/v citric acid is showing as orange colour ongraph ( Graph (C).Peak of stock solution of 5% w/v NaOH solution ( base containing sample) is showing deflection ( showing pink colourpeak on graph ( graph (B) ) which reflects that some part of equipment is leached out in base solution.Peak of stock solution of 5% w/v Citric acid solution ( acid containing sample) is showing deflection ( showing sea-greencolour peak on graph ( graph (D) ) which reflects that some part of equipment is also leached out in acid solution.IJRAR2001826International Journal of Research and Analytical Reviews (IJRAR) www.ijrar.org830
2020 IJRAR March 2020, Volume 7, Issue 1www.ijrar.org (E-ISSN 2348-1269, P- ISSN 2349-5138)Graph ( A )Graph ( B )Graph ( C )IJRAR2001826International Journal of Research and Analytical Reviews (IJRAR) www.ijrar.org831
2020 IJRAR March 2020, Volume 7, Issue 1www.ijrar.org (E-ISSN 2348-1269, P- ISSN 2349-5138)Graph ( D )8. CONCLUSION:After completing the procedure of passivation and analysis by UV spectroscopy. So, to sum it up all the observations andOn comparing the both, leaching was more observed in acidic solution ( Graph (E) as compared to basic solution ( graph(F) ).Graph ( E )Graph (F)IJRAR2001826International Journal of Research and Analytical Reviews (IJRAR) www.ijrar.org832
2020 IJRAR March 2020, Volume 7, Issue 1www.ijrar.org (E-ISSN 2348-1269, P- ISSN 2349-5138)10.REFERENCES:1)Benvenuti marco, “Validation, qualification and Calibration in a pharmaceutical”, SGS lifesciences services, Italy, 20142)ChoudharyAnkur, “Qualification of systems and equipments in pharmaceuticals”, Pharmaguideline, 20103)Fuchs John, “What is Passivation”,Techblog.20124)Choudhary Sachin, “Passivation of stainless steel in pharmaceuticals”, pharmapathway5)Wellspring pharma sciences, “What are IQ, OQ and PQ and why they are required”,Canada, 2014.6)Kessel k, 2016, Alternative to Nitric Acid Passivation, National Aeronautics and Space Administration, Kennedy Space Center, FL. KSC-E-DAA-TN360787)Lewis. P. Kolody M, and J.Curran, 2013a, Alternative to Nitric Acid Passivation, Department of Defense Corrosion Conference, 2013, Fort Meade MD. KSC-2013-2808)Lewis. P. Kolody M, and J.Curran, 2013b, Alternative to Nitric Acid Passivation of Stainless Steel Alloys, Department of Defense Corrosion Conference, 2013, Fort Meade MD.KSC-2013-2819)Drew C. Coleman and Daryl L. Roll,” CORROSION INVESTIGATION OF PHARMACEUTICAL CLEAN STEAM SYSTEMS”, May – June, 2017, armaceuticals/14)Coleman D.C. and R.W. Evans, “ Fundamentals of passivation and passivity in the Pharmaceutical industry” , Pharmaceutical Engineering 10, no. 2 (March-April 1990) ocessing16)http:/
From system inception, when a new CS generator and distribution tubing is first commissioned and energized, several potential factors for corrosion are present: In addition to clean steam, the CS generator begins to generate corrosion particles (class 1 rouge) that have the potential to migrate.