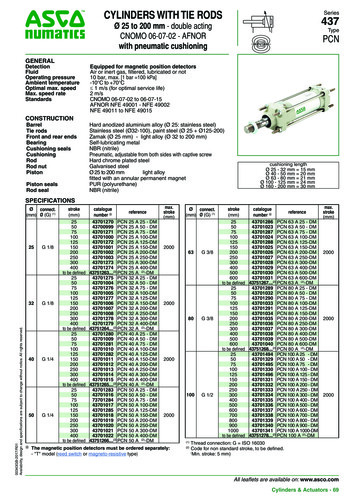
Transcription
MAGENTAMAGENTAMAGENTAMAGENTA Brand ofMethylTESTOSTERoneCapsules USP, 10 mgC-IIIDESCRIPTIONThe androgens are steroids that developand maintain primary and secondary malesex characteristics.Androgens are derivatives of cyclopen tanoperhydrophenanthrene. Endogenousandrogens are C-19 steroids with a side chainat C-17, and with two angular methyl groups.Testosterone is the primary endogenousandrogen. In their active form, all drugs in theclass have a 17-beta hydroxy group. 17-alphaalkylation (methylTESTOSTERone) increas es the pharmacologic activity per unit weightcompared to testosterone when given orally.MethylTESTOSTERone, a syntheticderivative of testosterone, is an androgenicpreparation given by the oral route in acapsule form. Each capsule contains 10 mgof MethylTESTOSTERone USP. It has thefollowing structural formula:OHCH3CH3HCH3HHO C20H30O2 M.W. hylTESTOSTERone occurs as white orcreamy white crystals or powder, which issoluble in various organic solvents but ispractically insoluble in water.Each capsule, for oral administration,contains 10 mg of MethylTESTOSTERone. Inaddition, each capsule contains the followinginactive ingredients: Corn starch NF, GelatinNF, FD&C Blue #1, FD&C Red #40.BLACK9410101100ct. Android Capsules Extended Container Label / PPSInside Insert 2.125” x 31.7185”Base Label 2.125” x 5”n/aAndroidCLINICAL PHARMACOLOGYEndogenous androgens are responsiblefor the normal growth and development ofthe male sex organs and for maintenance ofsecondary sex characteristics. These effectsinclude the growth and maturation of pros tate, seminal vesicles, penis, and scrotum.The development of male hair distribution,such as beard, pubic, chest, and axillary hair;laryngeal enlargement, vocal chord thicken ing, alterations in body musculature, and fatdistribution. Drugs in this class also causeretention of nitrogen, sodium, potassium,phosphorus, and decreased urinary excretionof calcium. Androgens have been reportedto increase protein anabolism and decreaseprotein catabolism. Nitrogen balance isimproved only when there is sufficient intakeof calories and protein.Androgens are responsible for the growthspurt of adolescence and for the eventualtermination of linear growth which is broughtabout by fusion of the epiphyseal growthcenters. In children, exogenous androgensaccelerate linear growth rates, but may causea disproportionate advancement in bonematuration. Use over long periods may resultin fusion of the epiphyseal growth centers andtermination of growth process. Androgenshave been reported to stimulate the pro duction of red blood cells by enhancing theproduction of erythropoietic stimulating factor.Dur ing exogenous administration ofandrogens, endogenous testosterone releaseis inhibited through feedback inhibition of pitu itary luteinizing hormone (LH). At large dosesof exogenous androgens, spermatogenesismay also be suppressed through feedbackinhibition of pituitary follicle stimulating hor mone (FSH).There is a lack of substantial evidencethat androgens are effective in fractures,surgery, convalescence and functional uter ine bleeding.PharmacokineticsTestosterone given orally is metabolizedby the gut and 44 percent is cleared by theliver of the first pass. Oral doses as high as400 mg per day are needed to achieve clini cally effective blood levels for full replacementtherapy. The synthetic androgen, methylTESTOSTERone, is less extensively metabolizedby the liver and has a longer half-life. It ismore suitable than testosterone for oraladministration.Testosterone in plasma is 98 percentbound to a specific testosterone-estradiolbinding globulin, and about 2 percent is free.Generally, the amount of this sex-hormonebinding globulin in the plasma will determinethe distribution of testosterone between freeand bound forms, and the free testosteroneconcentration will determine its half-life.About 90 percent of a dose of testosteroneis excreted in the urine as glucuronic andsulfuric acid conjugates of testosterone andits metabolites; and 6 percent of a dose isexcreted in the feces, mostly in the uncon jugated form. Inactivation of testosteroneoccurs primarily in the liver. Testosteroneis metabolized to various 17-keto steroidsthrough two different pathways. There areconsiderable variations of the half-life oftestosterone as reported in the literature,ranging from 10 to 100 minutes.In many tissues the activity of testos terone appears to depend on reduction todihydrotestosterone, which binds to cytosolreceptor proteins. The steroid-receptorcomplex is transported to the nucleus whereit initiates transcription events and cellularchanges related to androgen action.INDICATIONS AND USAGE1. MalesAndrogens are indicated for replace ment therapy in conditions associated witha deficiency or absence of endogenoustestosterone:1. Primar y hypogonadism (congenitalor acquired) — testicular failure dueto cryptorchidism, bilateral torsion,orchitis, vanishing testis syndrome; ororchidectomy.2. Hypogonadotropic hypogonadism(congenital or acquired) — gonado tropin or luteinizing hormone-releas ing hormone (LHRH) deficiency, orpituitar y hypothalamic injur y fromtumors, trauma, or radiation. (Appro priate adrenal cortical and thyroidhormone replacement therapy are stillnecessary, however, and are actuallyof primary importance.) If the aboveconditions occur prior to puber ty,androgen replacement therapy will beneeded during the adolescent yearsfor development of secondary sexualcharacteristics. Prolonged androgentreatment will be required to maintainsexual characteristics in these andother males who develop testosteronedeficiency after puberty.Safety and efficacy of Android (methyl testosterone) in men with “age-relatedhypogonadism” (also referred to as“late-onset hypogonadism”) have notbeen established.3. Androgens may be used to stimulatepuberty in carefully selected males withclearly delayed puberty. These patientsusually have a familial pattern ofdelayed puberty that is not secondaryto a pathological disorder; puberty isexpected to occur spontaneously at arelatively late date. Brief treatment withconservative doses may occasionallybe justified in these patients if they donot respond to psychological support.The potential adverse effect on bonematuration should be discussed withthe patient and parents prior to andro gen administration. An X-ray of thehand and wrist to determine bone ageshould be obtained every 6 months toassess the effect of treatment on theepiphyseal centers (see WARNINGS).2. FemalesAndrogens may be used secondarily inwomen with advancing inoperable metastatic(skeletal) mammary cancer who are 1 to5 years postmenopausal. Primary goals oftherapy in these women include ablationof the ovaries. Other methods of counter acting estrogen activity are adrenalectomy,hypophysectomy, and/or antiestrogen ther apy. This treatment has also been used inpremenopausal women with breast cancerwho have benefitted from oophorectomy andare considered to have a hormone-respon sive tumor. Judgment concerning androgentherapy should be made by an oncologist withexpertise in this field.CONTRAINDICATIONSAndrogens are contraindicated in menwith carcinomas of the breast or with knownor suspected carcinomas of the prostate,and in women who are or may becomepregnant. When administered to pregnantwomen, androgens cause virilization of theexternal genitalia of the female fetus. Thisvirilization includes clitoromegaly, abnormalvaginal development, and fusion of genitalfolds to form a scrotal-like structure. Thedegree of masculinization is related to theamount of drug given and the age of thefetus, and is most likely to occur in thefemale fetus when the drugs are given in thefirst trimester. If the patient becomes preg nant while taking these drugs, she should beapprised of the potential hazard to the fetus.WARNINGSIn patients with breast cancer, androgentherapy may cause hypercalcemia by stimu lating osteolysis. In this case, the drug shouldbe discontinued.Prolonged use of high doses of androgenshas been associated with the developmentof peliosis hepatis and hepatic neoplasmsincluding hepatocellular carcinoma. (SeePRECAUTIONS—Carcinogenesis). Peliosishepatis can be a life-threatening or fatalcomplication.Cholestatic hepatitis and jaundice occurwith 17-alpha-alkylandrogens at a relativelylow dose. If cholestatic hepatitis with jaundiceappears or if liver function tests becomeabnormal, the androgen should be discontin ued and the etiology should be determined.Drug-induced jaundice is reversible when themedication is discontinued.Geriatric patients treated with androgensmay be at an increased risk for the develop ment of prostatic hypertrophy and prostaticcarcinoma.There have been postmarketing reportsof venous thromboembolic events, includingdeep vein thrombosis (DVT) and pulmonaryembolism (PE), in patients using testosteroneproducts, such as methyltestosterone. Eval uate patients who report symptoms of pain,edema, warmth and erythema in the lowerextremity for DVT and those who present withacute shortness of breath for PE. If a venousthromboembolic event is suspected, discon tinue treatment with methyltestosterone andinitiate appropriate workup and management.Long term clinical safety trials have notbeen conducted to assess the cardiovascularoutcomes of testosterone replacement ther apy in men. To date, epidemiologic studiesand randomized controlled trials have beeninconclusive for determining the risk of majoradverse cardiovascular events (MACE), suchas non-fatal myocardial infarction, non-fatalstroke, and cardiovascular death, with theuse of testosterone compared to non-use.Some studies, but not all, have reportedan increased risk of MACE in associationwith use of testosterone replacement ther apy in men. Patients should be informedof this possible risk when deciding wheth er to use or to continue to use Android (methyltestosterone).Edema with or without congestive heartfailure may be a serious complication inpatients with preexisting cardiac, renal, orhepatic disease. In addition to discontinuationof the drug, diuretic therapy may be required.Gynecomastia frequently develops andoccasionally persists in patients being treatedfor hypogonadism.Androgen therapy should be used cau tiously in healthy males with delayed puberty.The effect on bone maturation should bemonitored by assessing bone age of thewrist and hand every 6 months. In children,androgen treatment may accelerate bonematuration without producing compensatorygain in linear growth. This adverse effectmay result in compromised adult stature.The younger the child the greater the risk ofcompromising final mature height.This drug has not been shown to be safeand effective for the enhancement of athleticperformance. Because of the potential riskof serious adverse health effects, this drugshould not be used for such purpose.
MAGENTAMAGENTAMAGENTAMAGENTA100ct. Android Capsules Extended Container Label / PPSInside Insert 2.125” x 31.7185”Base Label 2.125” x 5”n/aBLACK9410101PRECAUTIONSGeneralWomen should be observed for signs ofvirilization (deepening of the voice, hirsutism,acne, clitoromegaly and menstrual irreg ularities). Discontinuation of drug therapyat the time of evidence of mild virilism isnecessary to prevent irreversible virilization.Such virilization is usual following androgenuse at high doses. A decision may be madeby the patient and the physician that somevirilization will be tolerated during treatmentfor breast carcinoma.Information for the PatientThe physician should instruct patientsto report any of the following side effects ofandrogens:Adult orAdolescent Males: Too frequent or per sistent erections of thepenis.Any male adolescentpatient receiving andro gens for delayed puber ty should have bonedevelopment checkedevery six months.Women:Hoarseness, acne,changes in menstrualperiods or more hair onthe face.All Patients:Any nausea, vomiting,changes in skin color orankle swelling.Laboratory Tests1. Women with disseminated breast carcino ma should have frequent determinationof urine and serum calcium levels duringthe course of androgen therapy (SeeWARNINGS).2. Because of the hepatotoxicity associ ated with the use of 17-alpha-alkylatedandrogens, liver function tests should beobtained periodically.3. Periodic (every 6 months) X-ray exam inations of bone age should be madeduring treatment of prepubertal malesto determine the rate of bone maturationand the effects of androgen therapy on theepiphyseal centers.4. Hemoglobin and hematocrit should bechecked periodically for polycythemia inpatients who are receiving high doses ofandrogens.Drug Interactions1. Anticoagulants: C-17 substitutedderivatives of testosterone, such as meth androstenolone, have been reported todecrease the anticoagulant requirementsof patients receiving oral anticoagulants.Patients receiving oral anticoagulant ther apy require close monitoring, especiallywhen androgens are started or stopped.2. Oxyphenbutazone: Concurrentadministration of oxyphenbutazone andandrogens may result in elevated serumlevels of oxyphenbutazone.3. Insulin: In diabetic patients the meta bolic effects of androgens may decreaseblood glucose and insulin requirements.Drug/Laboratory Test InterferencesAndrogens may decrease levels of thyrox ine-binding globulin, resulting in decreasedtotal T4 serum levels and increased resinuptake of T3 and T4. Free thyroid hormonelevels remain unchanged, however, and thereis no clinical evidence of thyroid dysfunction.CarcinogenesisAnimal DataTestosterone has been tested by subcuta neous injection and implantation in mice andrats. The implant induced cervical-uterinetumors in mice, which metastasized in somecases. There is suggestive evidence thatinjection of testosterone into some strainsof female mice increases their susceptibilityto hepatoma. Testosterone is also known toincrease the number of tumors and decreasethe degree of differentiation of chemicallyinduced carcinomas of the liver in rats.Human DataThere are rare reports of hepatocellularcarcinoma in patients receiving long-termtherapy with androgens in high doses. With drawal of the drugs did not lead to regressionof the tumors in all cases.Geriatric patients treated with androgensmay be at an increased risk for the develop ment of prostatic hypertrophy and prostaticcarcinoma.PregnancyTeratogenic effects.Pregnancy Category X (See CONTRA INDICATIONS).Nursing MothersIt is not known whether androgens areexcreted in human milk. Because many drugsare excreted in human milk and because ofthe potential for serious adverse reactions innursing infants from androgens, a decisionshould be made whether to discontinuenursing or to discontinue the drug, takinginto account the importance of the drug tothe mother.Pediatric UseAndrogen therapy should be used verycautiously in children and only by specialistswho are aware of the adverse effects onbone maturation. Skeletal maturation mustbe monitored every six months by an X-rayof hand and wrist (See INDICATIONS ANDUSAGE and WARNINGS).ADVERSE REACTIONSEndocrine and UrogenitalFemale: The most common side effects ofandrogen therapy are amenorrhea and othermenstrual irregularities, inhibition of gonad otropin secretion and virilization, includingdeepening of the voice and clitoral enlarge ment. The latter usually is not reversibleafter androgens are discontinued. Whenadministered to a pregnant woman andro gens cause virilization of external genitaliaof the female fetus.Male: Gynecomastia, and excessivefrequency and duration of penile erections.Oligospermia may occur at high dosages(see CLINICAL PHARMACOLOGY).Skin and appendages: Hirsutism, malepattern baldness, and acne.Cardiovascular Disorders: myocardialinfarction, strokeFluid and Electrolyte Disturbances:Retention of sodium, chloride, water, potas sium, calcium and inorganic phosphates.Gastrointestinal: Nausea, cholestaticjaundice, alterations in liver function tests,rarely hepatocellular neoplasms and peliosishepatis (see WARNINGS).Hematologic: Suppression of clottingfactors II, V, VII, and X, bleeding in patientson concomitant anticoagulant therapy andpolycythemia.Nervous System: Increased or decreasedlibido, headache, anxiety, depression, andgeneralized paresthesia.Metabolic: Increased serum cholesterol.Vascular Disorders: venous thrombo embolism.Miscellaneous: Rarely anaphylactoidreactions.To repor t SUSPECTED ADVERSEREACTIONS, contact Valeant Pharma ceuticals North America LLC at 1-800 321-4576 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.DRUG ABUSE AND DEPENDENCEMethylTESTOSTERone Capsules areclassified as a schedule III ControlledSubstance under the Anabolic Steroids Actof 1990.OVERDOSAGEThere have been no reports of acuteoverdosage with the androgens.DOSAGE AND ADMINISTRATIONPrior to initiating Android (methyltestos terone), confirm the diagnosis of hypogo nadism by ensuring that serum testosteroneconcentrations have been measured in themorning on at least two separate days andthat these serum testosterone concentrationsare below the normal range.MethylTESTOSTERone capsules areadministered orally. The suggested dosagefor androgens varies depending on the age,sex, and diagnosis of the individual patient.Dosage is adjusted according to the patient’sresponse and the appearance of adversereactions.Replacement therapy in androgen-defi cient males is 10 to 50 mg of methylTESTOSTERone daily. Various dosage regimenshave been used to induce pubertal changesin hypogonadal males, some experts haveadvocated lower dosages initially, graduallyincreasing the dose as puberty progresseswith or without a decrease to maintenancelevels. Other experts emphasize that higherdosages are needed to induce pubertalchanges and lower dosages can be usedfor maintenance after puberty. The chrono logical and skeletal ages must be taken intoconsideration both in determining the initialdose and in adjusting the dose.Doses used in delayed puberty generallyare in the lower range of that given above,and for a limited duration, for example 4 to6 months.Women with metastatic breast carcinomamust be followed closely because androgentherapy occasionally appears to acceleratethe disease. Thus, many experts prefer touse the shorter acting androgen preparationsrather than those with prolonged activity fortreating breast carcinoma, particularly duringthe early stages of androgen therapy. Thedosage of methylTESTOSTERone for andro gen therapy in breast carcinoma in femalesis from 50-200 mg daily.HOW SUPPLIEDMethylTESTOSTERone capsules USP10 mg are red capsules imprinted “VRX0901” on both sections. They are availablein bottles of 100.NDC 0187-0902-01Store at 25 C (77 F); excursions permittedto 15 to 30 C (59 to 86 F) [see USP Con trolled Room Temperature].Manufactured for:Valeant Pharmaceuticals North America LLCBridgewater, NJ 08807 USABy:Valeant Canada LP1956 Bourdon StreetMontreal, Quebec, H4M 1V1Made in Canada9410101Revision 04/15Manufactured for:Valeant Pharmaceuticals North America LLCBridgewater, NJ 08807 USABy:Valeant Canada LP1956 Bourdon StreetMontreal, Quebec, H4M 1V1Made in Canada94101013 0187-0902-01 3NUMBER OF THE NDC CODENDC 0187-0902-01 (methylTESTOSTERone capsules, USP)100Capsules10 mgAndroidEach capsulecontains10 mgmethylTESTOSTERone,USPDispense in tight, light-resistant containers asdefined in USP.
PMS2613PMS3652.125"C.R.: 0.125"Non PrintingDielineMAGENTAUsual Dosage: See accompanying package insert.Store at 25 C (77 F); excursions permittedto 15 C to 30 C (59 F to 86 F) [see USPControlled Room Temperature].BLACKAndroid(methylTESTOSTERone capsules, USP)Each capsulecontains10 mgmethylTESTOSTERone,USPPATTERNVARNISH 10 mg100Capsules94101015"LEAFLET AREAALL TYPE SHOULDBE .125" AWAY FROMEDGE OF DIELINEMade in CanadaBy:Valeant Canada LP1956 Bourdon StreetMontreal, Quebec, H4M 1V1Manufactured for:Valeant Pharmaceuticals North America LLCBridgewater, NJ 08807 USADispense in tight, light-resistant containers asdefined in USP.MAGENTAMAGENTAMAGENTA100ct. Android Capsules Extended Container Label / PPSInside Insert 2.125” x 31.7185”9410100Base Label 2.125” x 5”n/aBASE LABELperf areaNDC 0187-0902-01
MAGENTAMAGENTAMAGENTAMAGENTA Brand ofMethylTESTOSTERoneCapsules USP, 10 mgC-IIIDESCRIPTIONThe androgens are steroids that developand maintain primary and secondary malesex characteristics.Androgens are derivatives of cyclopen tanoperhydrophenanthrene. Endogenousandrogens are C-19 steroids with a side chainat C-17, and with two angular methyl groups.Testosterone is the primary endogenousandrogen. In their active form, all drugs in theclass have a 17-beta hydroxy group. 17-alphaalkylation (methylTESTOSTERone) increas es the pharmacologic activity per unit weightcompared to testosterone when given orally.MethylTESTOSTERone, a syntheticderivative of testosterone, is an androgenicpreparation given by the oral route in acapsule form. Each capsule contains 10 mgof MethylTESTOSTERone USP. It has thefollowing structural formula:OHCH3CH3HCH3HHO C20H30O2 M.W. hylTESTOSTERone occurs as white orcreamy white crystals or powder, which issoluble in various organic solvents but ispractically insoluble in water.Each capsule, for oral administration,contains 10 mg of MethylTESTOSTERone. Inaddition, each capsule contains the followinginactive ingredients: Corn starch NF, GelatinNF, FD&C Blue #1, FD&C Red #40.BLACK100ct. Testred Capsules Extended Container Label / PPSInside Insert 2.125” x 29.7185”9410201Base Label 2.125” x 5”n/aTestredCLINICAL PHARMACOLOGYEndogenous androgens are responsiblefor the normal growth and development ofthe male sex organs and for maintenance ofsecondary sex characteristics. These effectsinclude the growth and maturation of pros tate, seminal vesicles, penis, and scrotum.The development of male hair distribution,such as beard, pubic, chest, and axillary hair;laryngeal enlargement, vocal chord thicken ing, alterations in body musculature, and fatdistribution. Drugs in this class also causeretention of nitrogen, sodium, potassium,phosphorus, and decreased urinary excretionof calcium. Androgens have been reportedto increase protein anabolism and decreaseprotein catabolism. Nitrogen balance isimproved only when there is sufficient intakeof calories and protein.Androgens are responsible for the growthspurt of adolescence and for the eventualtermination of linear growth which is broughtabout by fusion of the epiphyseal growthcenters. In children, exogenous androgensaccelerate linear growth rates, but may causea disproportionate advancement in bonematuration. Use over long periods may resultin fusion of the epiphyseal growth centers andtermination of growth process. Androgenshave been reported to stimulate the pro duction of red blood cells by enhancing theproduction of erythropoietic stimulating factor.Dur ing exogenous administration ofandrogens, endogenous testosterone releaseis inhibited through feedback inhibition of pitu itary luteinizing hormone (LH). At large dosesof exogenous androgens, spermatogenesismay also be suppressed through feedbackinhibition of pituitary follicle stimulating hor mone (FSH).There is a lack of substantial evidencethat androgens are effective in fractures,surgery, convalescence and functional uter ine bleeding.PharmacokineticsTestosterone given orally is metabolizedby the gut and 44 percent is cleared by theliver of the first pass. Oral doses as high as400 mg per day are needed to achieve clini cally effective blood levels for full replacementtherapy. The synthetic androgen, methylTESTOSTERone, is less extensively metabolizedby the liver and has a longer half-life. It ismore suitable than testosterone for oraladministration.Testosterone in plasma is 98 percentbound to a specific testosterone-estradiolbinding globulin, and about 2 percent is free.Generally, the amount of this sex-hormonebinding globulin in the plasma will determinethe distribution of testosterone between freeand bound forms, and the free testosteroneconcentration will determine its half-life.About 90 percent of a dose of testosteroneis excreted in the urine as glucuronic andsulfuric acid conjugates of testosterone andits metabolites; and 6 percent of a dose isexcreted in the feces, mostly in the uncon jugated form. Inactivation of testosteroneoccurs primarily in the liver. Testosteroneis metabolized to various 17-keto steroidsthrough two different pathways. There areconsiderable variations of the half-life oftestosterone as reported in the literature,ranging from 10 to 100 minutes.In many tissues the activity of testos terone appears to depend on reduction todihydrotestosterone, which binds to cytosolreceptor proteins. The steroid-receptorcomplex is transported to the nucleus whereit initiates transcription events and cellularchanges related to androgen action.INDICATIONS AND USAGE1. MalesAndrogens are indicated for replace ment therapy in conditions associated witha deficiency or absence of endogenoustestosterone:1. Primar y hypogonadism (congenitalor acquired) — testicular failure dueto cryptorchidism, bilateral torsion,orchitis, vanishing testis syndrome; ororchidectomy.2. Hypogonadotropic hypogonadism(congenital or acquired) — gonado tropin or luteinizing hormone-releas ing hormone (LHRH) deficiency, orpituitary hypothalamic injur y fromtumors, trauma, or radiation. (Appro priate adrenal cortical and thyroidhormone replacement therapy are stillnecessary, however, and are actuallyof primary importance.) If the aboveconditions occur prior to puber ty,androgen replacement therapy will beneeded during the adolescent yearsfor development of secondary sexualcharacteristics. Prolonged androgentreatment will be required to maintainsexual characteristics in these andother males who develop testosteronedeficiency after puberty.Safety and efficacy of Testred (methyl testosterone) in men with “age-relatedhypogonadism” (also referred to as“late-onset hypogonadism”) have notbeen established.3. Androgens may be used to stimulatepuberty in carefully selected males withclearly delayed puberty. These patientsusually have a familial pattern ofdelayed puberty that is not secondaryto a pathological disorder; puberty isexpected to occur spontaneously at arelatively late date. Brief treatment withconservative doses may occasionallybe justified in these patients if they donot respond to psychological support.The potential adverse effect on bonematuration should be discussed withthe patient and parents prior to andro gen administration. An X-ray of thehand and wrist to determine bone ageshould be obtained every 6 months toassess the effect of treatment on theepiphyseal centers (see WARNINGS).2. FemalesAndrogens may be used secondarily inwomen with advancing inoperable metastatic(skeletal) mammary cancer who are 1 to5 years postmenopausal. Primary goals oftherapy in these women include ablationof the ovaries. Other methods of counter acting estrogen activity are adrenalectomy,hypophysectomy, and/or antiestrogen ther apy. This treatment has also been used inpremenopausal women with breast cancerwho have benefitted from oophorectomy andare considered to have a hormone-respon sive tumor. Judgment concerning androgentherapy should be made by an oncologist withexpertise in this field.CONTRAINDICATIONSAndrogens are contraindicated in menwith carcinomas of the breast or with knownor suspected carcinomas of the prostate,and in women who are or may becomepregnant. When administered to pregnantwomen, androgens cause virilization of theexternal genitalia of the female fetus. Thisvirilization includes clitoromegaly, abnormalvaginal development, and fusion of genitalfolds to form a scrotal-like structure. Thedegree of masculinization is related to theamount of drug given and the age of thefetus, and is most likely to occur in thefemale fetus when the drugs are given in thefirst trimester. If the patient becomes preg nant while taking these drugs, she should beapprised of the potential hazard to the fetus.WARNINGSIn patients with breast cancer, androgentherapy may cause hypercalcemia by stimu lating osteolysis. In this case, the drug shouldbe discontinued.Prolonged use of high doses of androgenshas been associated with the developmentof peliosis hepatis and hepatic neoplasmsincluding hepatocellular carcinoma. (SeePRECAUTIONS—Carcinogenesis). Peliosishepatis can be a life-threatening or fatalcomplication.Cholestatic hepatitis and jaundice occurwith 17-alpha-alkylandrogens at a relativelylow dose. If cholestatic hepatitis with jaundiceappears or if liver function tests becomeabnormal, the androgen should be discontin ued and the etiology should be determined.Drug-induced jaundice is reversible when themedication is discontinued.Geriatric patients treated with androgensmay be at an increased risk for the develop ment of prostatic hypertrophy and prostaticcarcinoma.There have been postmarketing reportsof venous thromboembolic events, includingdeep vein thrombosis (DVT) and pulmonaryembolism (PE), in patients using testosteroneproducts, such as methyltestosterone. Eval uate patients who report symptoms of pain,edema, warmth and erythema in the lowerextremity for DVT and those who present withacute shortness of breath for PE. If a venousthromboembolic event is suspected, discon tinue treatment with methyltestosterone andinitiate appropriate workup and management.Long term clinical safety trials have notbeen conducted to assess the cardiovascularoutcomes of testosterone replacement ther apy in men. To date, epidemiologic studiesand randomized controlled trials have beeninconclusive for determining the risk of majoradverse cardiovascular events (MACE), suchas non-fatal myocardial infarction, non-fatalstroke, and cardiovascular death, with theuse of testosterone compared to non-use.Some studies, but not all, have reported anincreased risk of MACE in association withuse of testosterone replacement therapy inmen. Patients should be infor
Android (methylTESTOSTERone capsules, USP) 10 mg. 100 Capsules . Each capsule contains 10 mg USP . Dispense in tight, light-resistant containers as defined in USP. NDC 0187-0902-01 . 9410101. 3 0187-0902-01 3 NUMBER OF THE NDC CODE. 100ct.Android Capsules Extended Container Label / PPS Inside Insert 2.125" x 31.7185" Base Label 2.125 .