Transcription
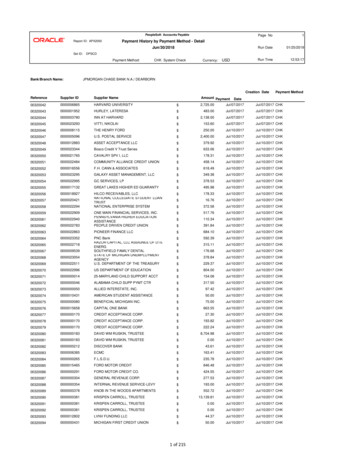
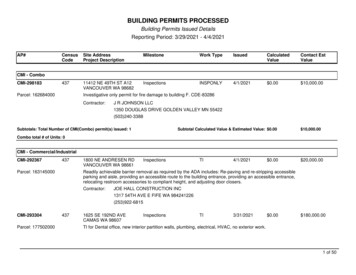
PL Detail-Document #301105 This Detail-Document accompanies the related article published in PHARMACIST’S LETTER / PRESCRIBER’S LETTERNovember 2014Magic Mouthwash RecipesIngredients1-11,a-eAmountDiphenhydramine 12.5 mg/5 mL 240 mLHydrocortisone60 mgNystatin powder6 millionunitsTetracycline1.5 gSwish and spit 5 mL QID.a.k.a. Mary’s Magic henhydramine 12.5 mg/5 mLa.k.a. Duke’s Magic Mouthwash4Amount60 mgSuspension30 mL ORPowder3 millionunitsQS 240 mLDistilled water160 mLHydrocortisone80 mgMaalox80 mLSwish and spit 5 mL QID.a.k.a. Weisman’s Philadelphia MouthwashDiphenhydramine 12.5 mg/5 mLHydrocortisoneNystatin suspensionTetracyclineDiphenhydramine 12.5 mg/5 mLNystatin suspensionMaaloxWaterDiphenhydramine 12.5 mg/5 mLHydrocortisoneNystatin suspensionTetracycline100 mL0.02 g4.8 mL200 mgDiphenhydramine 12.5 mg/5 mLPrednisone 5 mg/5 mLNystatin suspension1 part1 part1 partNystatin Susp. 100,000 U/mLLidocaine Viscous 2%Distilled WaterCrystal Light-Raspberry withAspartame crystals883.3 mL83.3 mL83.3 mL0.47 g1 part1 part1 part1 partDiphenhydramine 12.5 mg/5 mL 1 partViscous lidocaine 2%1 partMaalox1 partSwish and swallow 5 mL no more than Q4H.OR For radiation oncology mucositis; palliativecare:Swish, hold, and spit or swallow 30 mL Q2H.1Diphenhydramine 12.5 mg/5 mL240 mLHydrocortisone powder120 mg(wet with 1% CMCf to dissolve)Nystatin Suspension60 mLTetracycline 125 mg/5 mL120 mL(capsule dissolved in flavoredsyrup)CMCf 1%QS 480 mLSwish and swallow 10 mL TID.Diphenhydramine 12.5 mg/5 mLMylanta or MaaloxSucralfateSwish and spit or swallow 5 mLmeals and PRN.630 mL60 mL4gTID before180 mL0.072 g36 mL0.75 gCherry-flavored Kool-Aid mixed100 mLwith 2000 mL distilled water(sugar-free)Viscous lidocaine 2%100 mLNystatin suspension100 mLSwish and spit or swallow 15 mL QID. ORFor radiation oncology mucositis; palliativecare:Swish, hold, and spit or swallow 30 mL Q4H.1a.k.a. KoolstatMore. . .Copyright 2014 by Therapeutic Research Center3120 W. March Lane, Stockton, CA 95219 Phone: 209-472-2240 Fax: 209-472-2249www.PharmacistsLetter.com www.PrescribersLetter.com www.PharmacyTechniciansLetter.com
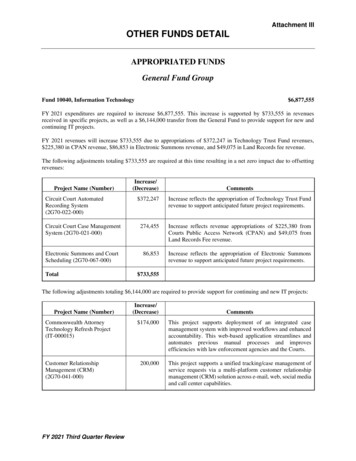
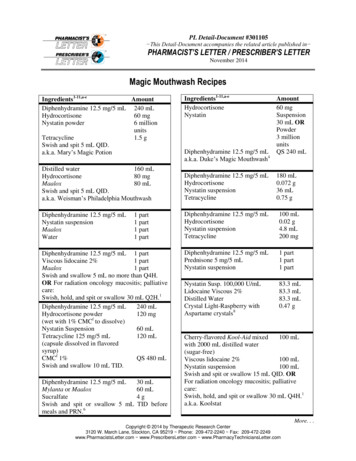
(PL Detail-Document #301105: Page 2 of 3)Ingredients1-11,a-eAmountHydrocortisone 100 mg/2 mL12 mL(Solu-Cortef)Nystatin suspension7.2 mLTetracycline 125 mg/5 mL12 mL(capsule dissolved in syrup)Diphenhydramine 12.5 mg /5 mL 150 mLSwish and swallow 10 mL QID.Viscous lidocaine 2%Hydrocortisone 100 mg/2 mL(Solu-Cortef)Nystatin suspensionMouth rinseDo not swallow.250 mL1g150 mLQS 500 mLDiphenhydramine 12.5 mg /5 mLDexamethasone 4 mg/mL injectionNystatin suspensionDistilled water QS to 200 mL8Swish and Spit 5 mL QID.120 mL0.56 mL40 mLViscous lidocaine 2%Cherry flavored Kool-Aid mixedwith 1500 mL of sterile water forirrigation (sugar-free)2000 mLQS3400 mLDiphenhydramine 12.5 mg/5 mLPrednisone 5 mg/5 mLNystatin suspension1 part1 part1 partViscous lidocaine 2%80 mLMylanta80 mLDiphenhydramine 12.5 mg/5 mL80 mLNystatin suspension80 mLPrednisolone 15 mg/5 mL80 mLDistilled water80 mLSwish, gargle, and spit 5 mL to 10 mL Q6HPRN.May be swallowed if esophagealinvolvement.10Viscous lidocaine 2%150 mLDiphenhydramine 12.5 mg/5 mL20 mLHydrocortisone (Solu-Cortef)100 mgTetracycline2 gramsNystatin suspension20 mLSwish, hold, and swallow 15 to 30 mL Q4-6H.1a.k.a. Mile’s SolutionIngredients1-11,a-eAmountViscous lidocaine 2%30 mLMaalox60 mLDiphenhydramine 12.5 mg/5 mL30 mLCarafate 1 g/10 mL40 mLSwish, gargle, and spit 5 mL to 10 mL Q6HPRN. May swallow if esophageal involvement.10Dexamethasone 0.5 mg/5 mL100 mLDiphenhydramine 12.5 mg/5 mL100 mLNystatin suspension60 mLTetracycline1500 mgSwish, gargle, and spit 5 mL to 10 mL Q6HPRN. May swallow if esophagealinvolvement.10a. Elixirs containing alcohol can cause stinging.Consider using injectable or powderformulation, crushing tablets, or openingcapsules in place of elixir formulation toavoid stinging.b. Some U.S. clinicians have found the newformulation of Kaopectate (i.e., containingbismuth) to solidify over a short period oftime when mixed with other ingredients. U.S.clinicians should consider this potentialproblem if utilizing recipes which useKaopectate in place of Maalox. CanadianKaopectate formulation does not containbismuth.c. Nystatin has not been shown to be effective intreating oral fungal infection associated withoral mucositis.11d. The use of corticosteroids, such ashydrocortisone or dexamethasone, has notbeen adequately studied to recommend itsinclusion in magic mouthwash.11e. In general, per USP standards, oral mixturescontaining water should have an expirationnot longer than two weeks (refrigerated) andmucosal mixtures containing water shouldhave an expiration of not longer than 30 days(room temp).12f. CMC Carboxymethylcellulose.Users of this PL Detail-Document are cautioned to use theirown professional judgment and consult any other necessaryor appropriate sources prior to making clinical judgmentsbased on the content of this document. Our editors haveresearched the information with input from experts,government agencies, and national organizations.Information and internet links in this article were current asof the date of publication.More. . .Copyright 2014 by Therapeutic Research Center3120 W. March Lane, Stockton, CA 95219 Phone: 209-472-2240 Fax: 209-472-2249www.PharmacistsLetter.com www.PrescribersLetter.com www.PharmacyTechniciansLetter.com
(PL Detail-Document #301105: Page 3 of 3)Project Leaders in preparation of this PL DetailDocument: Wan-Chih Tom, Pharm.D. (OriginalNovember 2009 version); Stacy A. Hester, R.Ph.,BCPS, Assistant Editor (November 2014 update)References1.2.3.4.5.The Erie St. Clair Palliative Care o.ca/PalliativeCareManagmenTool v3.2.pdf. (Accessed October 14, 2014).Anon.Slang terms and jargon can causemedication errors. Drugs & Therapy ecember 2005. Volume 19, 011/11/1005drugs-therapy-bulletin.pdf. (Accessed October 14,2014).Bulletin Board of Oral Pathology. University atBuffalo. .org/faqs/Pharmacist/faq DukesMagicMouthwash.htm.(Accessed October 14,2014).Hodgins C, Mosley M, Pola-Strowd M.Recommendationsfor thediagnosisandmanagement of recurrent aphthous stomatitis.2003. University of Texas at Austin, School ofNursing.6.Tarascon Pharmacopoeia. 2009 Library Edition.Ed. In Chief: Richard J. Hamilton. Jones &Bartlett. Sudbury, MA:164.7. Department of Pharmacy Services. Mount SinaiHospital. Toronto, Ontario MSG 1XS. October2009.8. Toronto Sunnybrook Regional Cancer CentrePharmacy. Toronto, Ontario M4N 3M5. October2009.9. Drug Information and Research Centre. OntarioPharmacist’s Association. October 2009.10. Randy Otterholt, DDS General h.html.(Accessed October 14, 2014).11. Chan A, Ignoffo RJ.Survey of topical oralsolutions for the treatment of chemo-induced oralmucositis. J Oncol Pharm Pract 2005;11:139-43.12. Chapter 795 Pharmaceutical Compounding-Nonsterile Preparations.The United StatesPharmacopeia and The National Formulary (USPNF).http://www.usp.org/sites/default/files/usp pdf/EN/gc795.pdf. (Accessed October 14, 2014).Cite this document as follows:PL Detail-Document, Magic Mouthwash Recipes.Letter/Prescriber’s Letter. November 2014.Pharmacist’sEvidence and Recommendations You Can Trust 3120 West March Lane, Stockton, CA 95219 TEL (209) 472-2240 FAX (209) 472-2249Copyright 2014 by Therapeutic Research CenterSubscribers to the Letter can get PL Detail-Documents, like this one,on any topic covered in any issue by going to m, or www.PharmacyTechniciansLetter.com
PL Detail-Document #301105 This PL Detail-Document gives subscribersadditional insight related to the Recommendations published in PHARMACIST’S LETTER / PRESCRIBER’S LETTERNovember 2014Prevention and Treatment of Oral MucositisOral mucositis is mucosal ulceration caused by chemotherapy or radiation treatment. Mucositis can affect not only the mouth, but also thepharyngeal, laryngeal, and esophageal areas.1 Mucositis is usually very painful and can be slow to heal.1-3 It can reduce an individual’s ability totolerate cancer treatment, maintain nutritional intake (e.g., drink, eat, swallow), or speak.1,4 Treatment guidelines have been developed for preventionand treatment of oral mucositis by the Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology(ISOO) 2014.pdf). The e5) as does the National Comprehensive Cancer Network (NCCN, http://www.nccn.org/JNCCN/PDF/mucositis 2008.pdf).Stomatitis is a term that is sometimes used interchangeably with mucositis, but is actually more general and describes any inflammatory condition oforal tissue.2 Canker sores are also different from oral mucositis. Their cause is unclear but they tend to be recurrent and triggered by factors such assmoking, stress, etc. For more info on treatment of canker sores, go to our PL Detail-Document, Treatment of Canker Sores. The following chartlists commonly asked questions about the treatment of oral mucositis. Keep in mind that the effectiveness of interventions may depend on factorssuch as treatments and the type of cancer being treated.QUESTIONHow can oral mucositis beprevented?ANSWER What nondrug therapiescan be recommended forpatients with oralmucositis? Proper oral hygiene can help minimize severity of oral mucositis.1,2Holding ice chips in the mouth, or cryotherapy, during treatment may help prevent mucositis in somesituations.1,3,12Oral zinc supplements may be helpful in some patients.1Rx palifermin (Kepivance) is FDA- and Health Canada-approved for prevention of oral mucositis in certainoncology patients.1,2 It can reduce severity and duration of mucositis.5Maintain proper oral hygiene, such as brushing the teeth with a soft-bristle toothbrush and flossing with gentleirrigation using a water flosser on a low setting.1,5Avoid irritating foods or beverages (e.g., dry, salty, acidic, hard, hot).5Rinsing frequently, such as every four hours or between medicated mouth rinse doses, with a bland solution, suchas one teaspoon of table salt in 32 oz. (1 L) of water (to make 0.9% sodium chloride) with or without one to twotablespoons of sodium bicarbonate, can be tried.1,2,5,12 This mixture can be used at room temperature orrefrigerated. The patient should rinse and swish then spit.2 (Note that experts suggest that patients who use wellwater make their salt solution with bottled water instead.)More. . .Copyright 2014 by Therapeutic Research Center3120 W. March Lane, Stockton, CA 95219 Phone: 209-472-2240 Fax: 209-472-2249www.PharmacistsLetter.com www.PrescribersLetter.com www.PharmacyTechniciansLetter.com
(PL Detail-Document #301105: Page 2 of 5)QUESTIONWhat are somecommercially availabletreatments for oralmucositis?ANSWER What are some commoningredients ofcompounded “magicmouthwash”? What is therationale for these? How effective is magicmouthwash? Continued An OTC antacid liquid (e.g., Amphojel) or film-forming agent (e.g., Zilactin) can be tried.2Rx oral protectants (e.g., Episil, Gelclair [alcohol-free], and MuGard-all U.S. only) may be more convenient thanOTC products.2 However, they can cost 100/week or more.5Commercial kits for compounding “magic mouthwash” are available in the U.S. These include:o First-Mouthwash BLM (diphenhydramine, lidocaine, aluminum/magnesium hydroxide)o First-BXN Mouthwash (diphenhydramine, lidocaine, nystatin)o First-Duke’s Mouthwash (diphenhydramine, hydrocortisone, nystatin)o First-Mary’s Mouthwash (diphenhydramine, hydrocortisone, nystatin, tetracycline)Magic mouthwash kits might be easier to e-prescribe and bill for than compounded products. However, kits aretypically about three times more expensive than mixing the mouthwash from scratch using individual ingredients(approximately 25 to 40 vs 5 to 15), and kits only come in specific combinations and concentrations.6The logic behind “magic mouthwash” is to combine ingredients with different mechanisms of action.5 There arenumerous magic mouthwash formulations. Most have at least three ingredients. Recipes may contain acombination of an antibiotic (to reduce the bacterial flora around the lesion), antihistamine (for local anestheticeffect), antifungal (to stop any fungal growth), steroid (to reduce inflammation), a local anesthetic/pain reliever, oran antacid (to enhance coating of the ingredients on the mouth).7Common ingredients of magic mouthwash recipes include viscous lidocaine, diphenhydramine, milk of magnesia,kaolin with pectin, and aluminum/magnesium hydroxide.2The most popular magic mouthwash formulation includes viscous lidocaine and diphenhydramine plusaluminum/magnesium hydroxide to help ingredients coat the mouth.There is a lack of controlled studies to evaluate the efficacy of the many different magic mouthwash recipes.Whether one recipe is more effective than another is unknown.8,12The 2004 guidelines for the treatment of oral mucositis suggest that magic mouthwashes (with variouscombinations of viscous lidocaine, benzocaine, milk of magnesia, kaolin-pectin, chlorhexidine, ordiphenhydramine) are no better than normal saline solution in pain relief.9 In addition, a Cochrane review foundmagic mouthwash (containing lidocaine, diphenhydramine, and aluminum hydroxide) to be ineffective inshortening the healing time of oral mucositis related to cancer therapies.10 There is also concern about theabsorption of anesthetics such as lidocaine when used on damaged mucosa.9Although frequently used as an ingredient of magic mouthwash, nystatin has not been shown to be effective intreating oral fungal infection associated with mucositis.7 It is also suggested that the high sugar content of nystatinsuspension may feed the fungus.8Corticosteroids have not been studied adequately to be recommended as an ingredient of magic mouthwash, andMore. . .Copyright 2014 by Therapeutic Research Center3120 W. March Lane, Stockton, CA 95219 Phone: 209-472-2240 Fax: 209-472-2249www.PharmacistsLetter.com www.PrescribersLetter.com www.PharmacyTechniciansLetter.com
(PL Detail-Document #301105: Page 3 of 5)QUESTIONANSWERcontinuedHow effective is magicmouthwash? Where can I find recipesfor magic mouthwash? What are some tips forcompounding magicmouthwash? What are some tips forbilling compounded magicmouthwash products? Continuedthere’s concern that long-term use may lead to oral candidiasis.Despite the lack of evidence that magic mouthwashes work in decreasing the pain associated withchemotherapy/radiation-induced mucositis, canker sores, or other oral pain conditions, many patients andprescribers continue to use them. There is a need to standardize the ingredients used to compound magicmouthwash in order to fully evaluate efficacy.We have a number of different recipes in our PL Chart, Magic Mouthwash Recipes.Most formulations are used every four to six hours with instructions to hold in the mouth for one to two minutesthen spit out or swallow. (Those with lidocaine should be spit out.)12 Patients should be instructed not to eat ordrink for 30 minutes after use.7Focal application should be used when possible, instead of widespread topical administration.2When compounding these mixtures, try to avoid using elixir formulations as the alcohol content can causestinging. Consider injectable or bulk powder formulations, crushed tablets, or opened capsules if needed.In some cases injectable formulations are used in place of elixirs. Some U.S. clinicians have found the newformulation of Kaopectate (i.e., containing bismuth) to solidify over a short period of time when mixed with otheringredients. U.S. clinicians should consider this potential problem if utilizing recipes which use Kaopectate in theplace of Maalox. Canadian Kaopectate formulation does not contain bismuth.The combination of lidocaine and sucralfate in magic mouthwash may not be compatible in some recipes. Someclinicians report the formation of a thick gel when the two ingredients are mixed.Prior to dispensing magic mouthwash, pharmacists should verify the formula and patient allergies. Patients shouldbe counseled regarding the proper use of magic mouthwash (e.g., to shake well before use, hold in mouth for aminute or two, whether to swallow or not, etc).Billing for magic mouthwash is not straightforward and varies among different pharmacies. There is no singleNDC number that can be used to bill for magic mouthwash mixed from individual ingredients. In addition, someof the ingredients used in the magic mouthwash are OTC products, which may not be covered by some insurances.Some pharmacists are left with the option of billing for a single prescription ingredient used for the compound andfor the full bottle used since billing a partial bottle is not allowed by insurance companies. Some pharmacists havethe patient pay cash. In some cases, the dispensing software allows the pharmacist to enter each ingredient usedand the cost of each ingredient for billing. In other cases, pharmacists may choose to bill each ingredientseparately as separate prescriptions.The magic mouthwash compounding kits each come with a single unique NDC number accounting for all theingredients, which can make billing less complicated in some cases. It may be easier to compound with these kitsMore. . .Copyright 2014 by Therapeutic Research Center3120 W. March Lane, Stockton, CA 95219 Phone: 209-472-2240 Fax: 209-472-2249www.PharmacistsLetter.com www.PrescribersLetter.com www.PharmacyTechniciansLetter.com
(PL Detail-Document #301105: Page 4 of 5)QUESTIONANSWERcontinuedWhat are some tips forbilling compounded magicmouthwash products? than having to measure and mix each individual ingredient. These compounding kits have also gone throughstability testing and have specified stability duration for an expiration date. Lastly, these mouthwashes have addedflavors and may be better tasting than mouthwashes compounded from scratch. Check insurance coverage beforeassembling the kit. Medicare and Medicaid coverage may be spotty because these are compounding kits, notapproved drug products. However, some managed plans may still cover the kits.Prescribers should specify mouthwash kits by brand name or specify the magic mouthwash formula.What beyond-use dateshould be assigned tocompounded magicmouthwash products? Beyond-use dates of these mixtures can vary depending on the ingredients and their individual expiration dates. Ingeneral, per USP standards, if a mixture contains water and is a mucosal liquid, the beyond-use date should not belonger than 30 days (room temperature).7,11 Oral mixtures containing water should have an expiration not longerthan two weeks (refrigerated).11How should pain fromoral mucositis bemanaged? Start with topical anesthetics such as topical lidocaine, 0.5% doxepin mouth rinse, or diphenhydramine mouthrinse.1,2Keep in mind most magic mouthwash recipes contain a topical anesthetic.If topical anesthetics don’t provide relief, consider opioids such as an alcohol-free morphine solution to swish andswallow, transdermal fentanyl, PCA morphine, etc.1,2,5What treatments for oralmucositis should actuallybe avoided? Avoid sucralfate or chlorhexidine, because they aren’t likely to help.1,3,4Also avoid alcohol-based mouth rinses, which can increase pain.12Avoid using hydrogen peroxide solutions (e.g., 3% hydrogen peroxide diluted 1:1 with water or normal saline) formore than two days. These may help remove oral debris, but longer p
More. . . Copyright 2014 by Therapeutic Research Center 3120 W. March Lane, Sto