Transcription
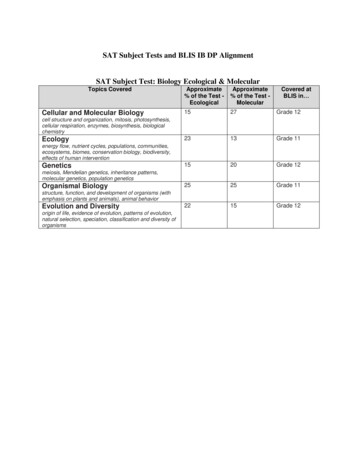
SAT Subject Tests and BLIS IB DP AlignmentSAT Subject Test: Biology Ecological & MolecularTopics CoveredCellular and Molecular BiologyApproximate% of the Test EcologicalApproximate% of the Test MolecularCovered atBLIS in 1527Grade 122313Grade 111520Grade 122525Grade 112215Grade 12cell structure and organization, mitosis, photosynthesis,cellular respiration, enzymes, biosynthesis, biologicalchemistryEcologyenergy flow, nutrient cycles, populations, communities,ecosystems, biomes, conservation biology, biodiversity,effects of human interventionGeneticsmeiosis, Mendelian genetics, inheritance patterns,molecular genetics, population geneticsOrganismal Biologystructure, function, and development of organisms (withemphasis on plants and animals), animal behaviorEvolution and Diversityorigin of life, evidence of evolution, patterns of evolution,natural selection, speciation, classification and diversity oforganismsCONTENT Approximate% of Test
SAT Subject Test: Math I & IITopics CoveredApproximate% of the Test Level 1Approximate% of the Test Level 210–1410–14Grade 1138–4248–52Grade 11Geometry and Measurement38–4228–32Grade 11Plane Euclidean/Measurement18–22-----Grade 11Coordinatelines, parabolas, circles, ellipses, hyperbolas, symmetry,transformations, polar coordinatesThree-dimensionalsolids, surface area & volume (cylinders, cones, pyramids,spheres, prisms), coordinates in three dimensions8–1210–14Grade 114–64–6Grade 11Trigonometryright triangles, identities, radian measure, law of cosines,law of sines, equations, double angle formulas6–812–16Grade 11Data Analysis, Statistics, and Probability6–106–10Grade 11 or 12(also coveredin IGCSEMaths)Number and OperationsCovered atBLIS in operations, ratio & proportion, complex numbers, counting,elementary number theory, matrices, sequences, series,vectorsAlgebra and Functionsexpressions, equations, inequalities, representation andmodeling, properties of functions (linear, polynomial,rational, exponential, logarithmic, trigonometric, inversetrigonometric, periodic, piecewise,recursive, parametric)mean, median, mode, range, interquartile range, standarddeviation, graphs and plots, leastsquares regression(linear, quadratic, exponential), probability
SAT Subject Test: ChemistryTopics CoveredStructure of MatterApproximate% of the TestCovered atBLIS in 25Grade 1116Grade 1114Grade 1214Grade 115Grade 126Grade 1112Grade 128Grades 11 &12Atomic Structure, including experimental evidence of atomicstructure, quantum numbers and energy levels (orbitals), electronconfi gurations, periodic trendsMolecular Structure, including Lewis structures, threedimensional molecular shapes, polarityBonding, including ionic, covalent, and metallic bonds,relationships of bonding to properties and structures;intermolecular forces such as hydrogen bonding, dipole-dipoleforces, dispersion (London) forcesStates of MatterGases, including the kinetic molecular theory, gas lawrelationships, molar volumes, density, and stoichiometryLiquids and Solids, including intermolecular forces in liquids andsolids, types of solids, phase changes, and phase diagramsSolutions, including molarity and percent by massconcentrations, solution preparation and stoichiometry, factorsaffecting solubility of solids, liquids, and gases, qualitative aspectsReaction TypesAcids and Bases, including Brønsted-Lowry theory, strong andweak acids and bases, pH, titrations, indicatorsOxidation-Reduction, including recognition of oxidation-reductionreactions, combustion, oxidation numbers, use of activity seriesPrecipitation, including basic solubility rulesStoichiometryMole Concept, including molar mass, Avogadro’s number,empirical and molecular formulasChemical Equation, including the balancing of equations,stoichiometric calculations, percent yield, and limiting reactantsEquilibrium and Reaction RatesEquilibrium Systems, including factors affecting position ofequilibrium (LeChâtelier’s principle) in gaseous and aqueoussystems, equilibrium constants, and equilibrium expressionsRates of Reactions, including factors affecting reaction rates,potential energy diagrams, activation energiesThermochemistryIncluding conservation of energy, calorimetry and specific heats,enthalpy (heat) changes associated with phase changes andchemical reactions, heating and cooling curves, randomness(entropy)Descriptive ChemistryIncluding common elements, nomenclature of ions andcompounds, periodic trends in chemical and physical properties ofthe elements, reactivity of elements and prediction of products ofchemical reactions, examples of simple organic compounds andcompounds of environmental concernLaboratoryIncluding knowledge of laboratory equipment,measurements, procedures, observations, safety, calculations,data analysis, interpretation of graphical data, drawingconclusions from observations and data
SAT Subject Test: PhysicsTopics CoveredMechanicsApproximate% of the TestCovered atBLIS in 36–42Grade 1118–24Grade 1215–19Grade 116–11Grade 116–11Grade 124–9Grades 11-128Grades 11 &12Kinematics, such as velocity, acceleration, motion in onedimension, and motion of projectilesDynamics, such as force, Newton’s laws, and staticsEnergy and Momentum, such as potential and kinetic energy,work, power, impulse, and conservation lawsCircular Motion, such as uniform circular motion and centripetalforceSimple Harmonic Motion, such as mass on a spring and thependulumGravity, such as the law of gravitation, orbits, and Kepler’s lawsElectricity and MagnetismElectric Fields, Forces, and Potentials, such as Coulomb’s law,induced charge, field and potential of groups of point charges, andcharged particles in electric fieldsCapacitance, such as parallel-plate capacitors and transientsCircuit Elements and DC Circuits, such as resistors, light bulbs,series and parallel networks, Ohm’s law, and Joule’s lawMagnetism, such as permanent magnets, fields caused bycurrents, particles in magnetic fields, Faraday’s law, Lenz’s lawWaves and OpticsGeneral Wave Properties, such as wave speed, frequency,wavelength, superposition, standing waves, and Doppler effectRefl ection and Refraction, such as Snell’s law and changes inwavelength and speedRay Optics, such as image formation using pinholes, mirrors, andlensesPhysical Optics, such as single-slit diffraction, double-slitinterference, polarization, and colorHeat and ThermodynamicsThermal Properties, such as temperature, heat transfer, specificand latent heats, and thermal expansionLaws of Thermodynamics, such as first and second laws,internal energy, entropy, and heat engine efficiencyModern PhysicsQuantum Phenomena, such as photons and photoelectric effectAtomic, such as the Rutherford and Bohr models, atomic energylevels, and atomic spectraNuclear and Particle Physics, such as radioactivity, nuclearreactions, and fundamental particlesRelativity, such as time dilation, length contraction, and massenergy equivalenceMiscellaneousGeneral, such as history of physics and general questions thatoverlap several major topicsAnalytical Skills, such as graphical analysis, measurement, andmath skillsContemporary Physics, such as astrophysics, superconductivity,and chaos theoryLaboratoryIncluding knowledge of laboratory equipment,measurements, procedures, observations, safety, calculations,data analysis, interpretation of graphical data, drawingconclusions from observations and data
SAT Subject Test: Chemistry Topics Covered Approximate % of the Test Covered at BLIS in Structure of Matter Atomic Structure, including experimental evidence of atomic structure, quantum numbers and energy levels (orbitals), electron confi gurations, periodic trends Molecular Structure, including Lewis structures, three-










