Transcription
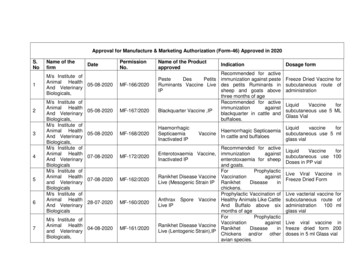
Approval for Manufacture & Marketing Authorization (Form-46) Approved in 2020S.NoName of thefirm1M/s Institute ofAnimal Health05-08-2020And VeterinaryBiologicals,234567M/s Institute ofAnimal HealthAnd VeterinaryBiologicals,M/s Institute ofAnimal HealthAnd VeterinaryBiologicals,M/s Institute ofAnimal HealthAnd VeterinaryBiologicalsM/s Institute ofAnimal Healthand VeterinaryBiologicalsM/s Institute ofAnimal HealthAnd 2020M/s Institute ofAnimal Health04-08-2020and VeterinaryBiologicals,MF-161/2020Name of the ProductapprovedIndicationRecommended for activePesteDesPetits immunization against pesteRuminants Vaccine Live des petits Ruminants inIPsheep and goats abovethree months of ageRecommended for activeimmunizationagainstBlackquarter Vaccine ,IPblackquarter in cattle andbuffaloes.HaemorrhagicSepticaemiaInactivated IPVaccineDosage formFreeze Dried Vaccine forsubcutaneous route ofadministrationLiquidVaccineforsubcutaneous use 5 MLGlass VialLiquidvaccineforHaemorrhagic Septicaemiasubcutaneous use 5 mlIn cattle and buffaloesglass vialRecommended for activeEnterotoxaemia Vaccine, immunizationagainstInactivated IPenterotoxaemia for sheepand goats.ForProphylacticRanikhet Disease Vaccine VaccinationagainstLive (Mesogenic Strain IP RanikhetDiseaseinchickens.Prophylactic Vaccination ofAnthrax Spore Vaccine Healthy Animals Like CattleLive IPAnd Buffalo above sixmonths of ageForProphylacticVaccinationagainstRanikhet Disease VaccineRanikhetDiseaseinLive (Lentogenic Strain),IPChickens and/or otheravian species.LiquidVaccineforsubcutaneous use 100Doses in PP vialLive Viral VaccineFreeze Dried ForminLive vacterial vaccine forsubcutaneous route ofadministration100 mlglass vialLive viral vaccine infreeze dried form 200doses in 5 ml Glass vial
8M/s Institute ofAnimal Health05-08-2020and VeterinaryBiologicalsMF-165/2020For active immunization LiquidvaccineforRabies Veterinary vaccineagainst rabies in dogs cats subcutaneousrouteinactivated (cell culture)IPand other mammal.single dose and 5 doses9M/s Biovet Pvt19-08-2020LtdMF-192/2020Combined foot and mouthdiseaseandHaemorrhagicSepticaemia Inactivated,vaccine Oil10M/s Brilliant Bio11-08-2020Pharma Pvt. LtdBulk-183/2020Foot and Mouth DiseaseConcentratedBulk,Antigen, inactivated IHS11M/s Biovet 7-08-2020LimitedMF-173/202013M/s Indovax Pvt15-07-2020LtdMF-124/202014M/s Indovax Pvt01-06-2020LtdMF-100/202015M/s Indovax Pvt18-03-2020LtdBulk-53/2020For active immunization ofanimals against foot andmouthDiseaseandhaemorrhagic SepticaemiaDisease InfectionFor active immunization ofBrucella abortus (strainanimals against brucellosis19) vaccine live IP (vet)diseaseFor prophylactic calf-hoodBrucella Abortus (Strainvaccinationagainst19) Vaccine,Live FreezeBrucella Aboruts S19 StrainDriedto Female Calves only.Forprotection againstNewcastle Disease byNewcastleDiseaseboosting up the immunity ofVaccine-MesogenicflockspreviouslyStrain, Inactivated IP intramuscular route ofadministration30ml:30mlpp vial 10doses90ml:100ml pp vial 300dosesFreeze dried vaccine forsubcutaneous route ofadministrationFreeze Dried Vaccine tobe reconstitution with thesupplied diluent 5 dosevialWater in oil emulsionvaccine in 1000 dosespresentation inject 0.5 mldose subcutaneously fvaccinerouteofSalmonellavaccine commercial layers andadministrationforinactivated ipbreeders against Fowlsubcutaneous injectionTyphoid Disease.1000 dosesNewcastleDiseasevaccine bulk live (R2BStrain )
16M/s Brilliant Bio12-05-2020Pharma Pvt LtdMF-79/202017M/s Brilliant Bio04-09-2020Pharma Pvt. LtdBulk-209/202018M/sSanvitaBiotechnologies 10-09-2020Pvt imited,MF-275/2020Forprophylacticvaccination against bothFoot and mouth Disease foot and mouth disease and Biological (Vaccine) PackHaemorrhagicpasteurella multocida in of 20Ml, 30Ml, 50mL,Septicaemia Vaccinecloven hoofed animals 100mL, & 200 ml.such as cattle buffaloes,sheep and goats.RabiesVeterinaryConcentratedBulk,Antigen Inactivated, HIS.Freeze dried vaccine forFor immunization againstBrucella Aboruts S19subcutaneous route ofbrucellosisdiseaseinVaccine Frozen vaccine to beTo be used against theMarek’s Vaccine Live IPusedsubcutaneouslymarek’s disease in day oldFrozen SB1 Strainafter reconstitution withchicks.diluentFrozen vaccine to beTo be used against theMarek’s Disease Vaccineusedsubcutaneouslymarek’s disease in day oldlive ip frozen Sb1 strainafter reconstitution withchicks.diluent.Infectiousavian To be used in breeder hensencephalomyelitis vaccine to give broad protection Emulsion for injectioninactivated van roekel againstavian wateR/ oil/ waterstrainencephalomyelitis virusCalciumphosphateNewcastleDisease To be used againstcoupled freeze driedVaccine,LiveNano Newcastlediseaseinvaccine for oculo- nasal(Lasota Strain)poultryroute of administration
Approval for Import & Marketing Authorization (Form-45) Approved in 2020S.No12345Name of thefirmM/sSTCInternationalLLPM/s Uni-ChemieInternational,M/s Uni-ChemieInternationalDatePermissionNo.Name of the Productapproved13-08-2020Bulk-182/2020Tylvalosin Tartrate 80 %17-08-2020Bulk-188/2020Zinc Bacitracin IP (Vet)13-07-2020Bulk-131/2020Tiamulin Fumarate 80%Premix et15-09-2020India Pvt LtdIndicationDosage fectious Bronchitis M-41Strain, Infectious BursalDisease Virus VP2 Proteinand viral arthritis Reo VirusS1133 Strain, Inactivatedoil emulision vaccineFor active immunization ofchickenstoreducemortality clinical signs andlesions associated withinfections caused by onchitisinfectious bursal diseaseand viral arthritis reo virusWater in oilemulsionvaccine 500mlHDPE Bottles(1000 doses )IMP-219/2020Treatment and control ofpoultryreomitesdermanyssus gallinae orFluralaner10mg/mlnorthernfowlmitesolution exzolt solutionornothonyssus sylviaruminfestationinpulletsbreeders and layer hensOralsolutionlight yellowishbrown solutionforuseindrinking water )
vet12-10-2020India Pvt LtdM/sIntervet15-09-2020India Pvt LtdFor active immunization ofchickenstoreducemortality clinical signs andlesions associated withinfections caused by nchitisinfectious bursal diseaseand viral arthritis Reo virusWater in oilemulsionvaccine 500 mlHDPE Bottles(1000 dosesIMP-246/2020For active Immunization ofLivevaccineagainst layers in order to inducesalmonellagallinarum immunityagainstorganism strain 9Rsalmonellagallinaruminfectionlive vaccine infreezedriedpellet form utionpresentation1000 dosesIMP-219/2020This drug is treatment andcontrol of poultry red miteddermanyssus gallinae ormg/mlnorthernfowlmiteornothonyssus sylviaruminfestrationinpulletsbreeders and layer hens.Oralsolutionlight yellowishbrown solutionforuseindrinking nfectious bronchitis M41Strain, Infectious bursalDisease virus VP2 proteinand viral arthritis reo viruss1133 strain inactivated oilemulsionvaccinequadractin vp2Floralanersolution.10
09M/s Hipra India23-11-2020Pvt. Ltd.,IMP-279/2020For active immunization ofchicks from 1 day of ageagainst coccidiosis causedLive Attenuated Vaccine, by Eimeria acervulina,Avian Coccidiosis (Evant), Eimeria Maxima, EimeriaMitis, Eimeria Praecox andeimeria tenella to reduceclinical signs,Suspension 35ml)and10,000doses (70ml)
Approval for Import & Marketing Authorization (Form-45) Approved in 2020 S. No Name of the firm Date Permission No. Name of the Product approved Indication Dosage form 1 M/s STC International LLP 13-08-2020 Bulk-182/2020 Tylvalosin Tartrate 80 % 2 M/s Uni-Chemie International, 17-08-2020 Bulk-188/2020 Zinc Bacitracin IP (Vet) 3










