Transcription
A Look atColumn ChoicesMark PowellApplications EngineerColumns and SuppliesTechnical SupportConfidentiality LabelApril 8, 20151
OverviewWhat to consider when choosing a column? Column chemistry Silica surface Bonded phase End-capping Particle size options Totally porous Superficially porous Method conditions Column lifetime
Silica Column CharacteristicsSurface area Pore sizeParticle sizeSurface chemistry iCH3CH3CH3OSiCH3Confidentiality LabelApril 8, 20153
Pore SizeSmall molecules 80 – 120 Å Maximizes loading and retentionPeptides, proteins, other large biomolecules 120 Å (Peptides) 300 Å to 450 Å (or higher) Maintain high efficiencyConfidentiality LabelApril 8, 20154
Pore SizeConfidentiality LabelApril 8, 20155
Particle TechnologiesYear(s) ofAcceptanceParticle SizeMost PopularNominal SizePlates / 15cm100µm200196757µm .5µm22,000 2µm 30,0001950’s20032007/2008 2.7 µm32,000
Particle Technologies2.7 and 4 µm superficially porous
Superficially Porous Column TechnologiesPoroshell 120 columns: Efficiency 90% of sub-2 µm Pressure 40-50% of sub-2 µm N 2X 3.5 µm (totally porous) dp 2.7µm 2 µm frit to reduce clogging Plimit 600 bar for HPLC or UHPLC Particles 1.7 μm solid core 0.5 μm diffusion path 2.7 μm total diameter0.5um1.7um0.5um
Comparing Efficiency and Pressure with DifferentTypes of ColumnsParticle Size/Type Pressure Efficiency LC Compatibility5 µmTotally Porous80 bar5,000All 400 barinstruments3.5 µmTotally Porous123 bar7,800All 400 barinstruments2.7 µmPoroshell 120180 bar12,000All LCs/UHPLCs(up to 600 bar)12,500All LCs/UHPLCs(up to 1200 bar)1.8 µmTotally Porous285 barColumns: 4.6 x 50mm, Mobile Phase: 60% ACN:40% Water Flow Rate: 2 mL/min
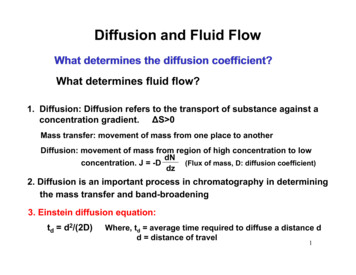
Efficiency Improvementwith Superficially Porous Particlesvan Deemter equation:h A B / C B A term – eddy diffusion andflow distribution particle size & packing qualityimportant narrow particle size distributionh B term – longitudinal diffusionCCAASeparation Speed (v) Slightly lower due to longitudinaldiffusion reduction C term – mass transfer shorter diffusion paths better with superficially porousparticles more effect on large moleculesLower h higher efficiency!Agilent Confidential4/8/201510
Comparison of Particle Size DistributionsTotally Porous and Poroshell 120 Particles
Analyte Mass Transfer Improvements through LowerDiffusionTotally Porous Totally porous particles diffusion throughout particle Poroshell 120 diffusion limited to outer shellvan Deemter equation:h A B / C Superficially Porous Results: Lower C term Higher efficiency And Higher flow rate with Minimal impact on efficiency
Pore Size and EfficiencyConfidentiality LabelApril 8, 201513
Making a Poroshell ParticleMake the solid coreApply the porous shell Smooth surfaceTight particle size distributionTightly packed column bedHigher efficiencySingle coacervation stepHigh yieldsBetter reproducibilityApply the bondedphaseConfidentiality LabelApril 8, 201514
Silica Particle SurfaceConfidentiality LabelApril 8, 201515
Typical Stationary Phase Bonding and EndcappingReactionCH 3CH 3OHOHOHOH OCH 3ClSiRCH 3R C8, C18, etc .SiROH CH 3 OH CH 3OSiCH 3RSi RCH 3OHCH 3O Si CH 3CH 3CH 3O Si ROCH 3ClSiCH 3(TMS)CH 3Month ##, 200XGroup/Presentation TitleAgilent Restricted
The Surface of Silica iSiAssociatedSilanolsdecreasing acidityOH MSurface Metal MSiInternal Metal(activated silanol)(most acidic)Month ##, 200XGroup/Presentation TitleAgilent Restricted
Potential Secondary InteractionsIon-exchangeSiO Na R3NH 1.SiO N R3 Na Ionized silanols (SiO-) will ion-exchange with protonated bases (R3NH )which can cause tailing and method variability. This occurs most often atmid pH where silanols are ionized.Hydrogen bonding-SiOH RCOO2.-SiO . . . H . . . OOCRUnprotonated acids can compete for H with protonated silanols.This can occur at low pH.Month ##, 200XGroup/Presentation TitleAgilent Restricted
Highly Purified Zorbax Rx-SilOriginal ZORBAX, 1973 and other type A silicas basic compounds can tailConditions: Flow Rate: 2.0 mL / min.Mobile Phase: 5% 2-Propanol in HeptaneZORBAX Rx-Sil, 1987 and other Type Bsilicas basic compounds have less tailing lower effective silanol pKaMonth ##, 200XGroup/Presentation TitleAgilent Restricted9
Poroshell 120 Column ChemistriesMultiple bonded phases for flexibility in method developmentPoroshell 120 SB-AqPoroshell 120 EC-C18 and C8 Robust end-capped C18/C8 forbest peak shape at pH 2-9 Proprietary bonded phase is anexcellent choice for polar analytesPoroshell 120 Stablebond C18and C8Poroshell 120 Bonus-RP Robust chemistries for pH 2 Non-endcapped for alternateselectivity Embedded polar group provides uniqueselectivity for polar compoundsPoroshell 120 EC-CNPoroshell HPH-C18 and HPH-C8 Flexible end-capped CN chemistry fornormal and reversed-phase separations Poroshell 120 HILICLong lifetime at high pHPoroshell 120 Phenyl-Hexyl Excellent choice for pi-pi interactions Selectivity similar to phenyl, diphenyl, orother phenyl-hexyl columns Bare silica HILIC for use in hydrophilicinteraction chromatography of polarmoleculesPoroshell 120 PFP Pentafluorophenyl chemistry fororthogonal selectivity relative to C18Agilent Confidential4/8/201520
Porshell 120 Column ChemistriesBonded ylSB-AqBonus-RPHILICEC-CNPFPPore Size (Å)120120120120100100120120120120120120Temp. Limit ( C)606090806060608060606060pH Range2-82-81-81-83 - 113 - eDoubleDoubleNoTripleNoDoubleYesCarbon Load 3.55.1Surface Area iality LabelApril 8, 201521
Why So Many Phase Chemistries?Resolution7.006.00Increasing N5.00Increasing AlphaIncreasing k'4.00Rs N½/4 ( -1)/ k’/(k’ ty Impacts Resolution Most Change bonded phaseTypical Method Development Parameters Change mobile phase Plates are easiest to increaseAgilent Confidential4/8/201522
Method Development SchemeChanging selectivity to improve resolution:Mobile phase (1st choice to change because it’s easy) Mobile phase – organic modifier (ACN, MeOH etc.)Mobile phase – pH – over a wide pH range – pH 1-12 if neededBonded phase (optimization for robust methods) Phases other than C18/C8Phenyl-Hexyl, Polar-embedded, CN, PFP
Why Is Changing the Bonded Phase Effective? Differences in interactions between polar and non-polar compounds. Other types of interactions with a bonded phase can be exploited (pi -piinteractions etc.) These all change with bonded phase! Changing the bonded phase can improve selectivity/resolution, reduceanalysis timeWhen you use Poroshell 120 columns the comparison of bonded phasescan be done quickly!! Multiple column choices available with different high speed technologies make this easy
Change in Retention with pH for IonizableCompounds is Compound-DependentMore retention for non-charged
Superficially Porous Column Technologies Efficiency 90% of sub-2 µm Pressure 40-50% of sub-2 µm N 2X 3.5 µm (totally porous) d p 2.7µm 2 µm frit to reduce clogging P limit 600 bar for HPLC or UHPLC Particles 1.7 μm solid core 0.5 μm diffusion path 2.7 μm total diameter Poroshell .