Transcription
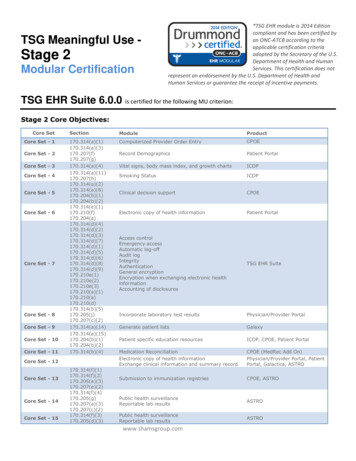
Stage 2 Meaningful Use:Menu Objectives andClinical Quality MeasuresJames R. Christina, DPMDirector Scientific AffairsAPMA
What Stage Am I In?22
CMS Proposed Rule On May 20, 2014 CMS and Office of the NationalCoordinator (ONC) released a notice of proposedrulemaking (NPRM) that would allow providersparticipating in the EHR Incentive Programs to use the 2011Edition of certified electronic health record technology(CEHRT) for calendar and fiscal year 2014. The NPRM will grant flexibility to providers who areexperiencing difficulties fully implementing 2014 EditionCEHRT to attest this year. The proposed rule would allowproviders to use EHRs that have been certified under the2011 Edition, a combination of the 2011 and 2014 Editions,or the 2014 Edition. Beginning in 2015, all eligible providers would be required toreport using 2014 Edition CEHRT.
If you can attest to not being able to‘fully implement 2014 CEHRT” thenyou have the following optionsIf youwerescheduledtodemonstrate:Stage 1 in2014Stage 2 in2014You would be able to attest for MU:Using 2011 Using 2011 & Using 2014Edition2014 EditionEditionCEHRT toCEHRT toCEHRT todo:do:do:2013 Stage 1objectives andmeasures2013 Stage 1-orobjectivesand measures 2014 Stage 1objectives andmeasures2013 Stage 1objectives andmeasures-OR2013 Stage 1 2014 Stage 1objectivesobjectives andand measuresmeasures-ORStage 2objectives andmeasures2014 Stage 1objectivesand measures2014 Stage 1objectivesand measures-ORStage 2objectivesand measures
FACTS ABOUT PROPOSED RULE: This is a proposed rule meaning that there is a 60 day comment period. APMA will comment on theproposed rule, particularly stating that there should not be the need to attest to inability to “fullyimplement” but all providers should have the option of choosing what CEHRT they want to use toattest. The comment period will end on July 21, 2014. CMS will probably not issue the final rule on this until around September 1, 2014. That will mean whenthe final rule comes out there will be only one calendar quarter (Oct-Dec) left to meet therequirements for MU for 2014. CMS has indicated that if you feel that you will attest to not being able to fully implement 2014 CEHRTand you are able to meet the MU requirements in the proposed rule during one of the calendarquarters prior to the final rule, you will be able to attest to that calendar quarter once the rule isfinalized. So for example, if you were scheduled to demonstrate Stage 1 or Stage 2 of MU in 2014 andduring one of the calendar quarters you were still using your 2011 CEHRT, if you met the 2013 Stage 1objectives and measures, you would be able to attest to that quarter once the final rule was published. Note that the options in the table listed depend on whether you were scheduled to report on Stage 1or Stage 2 in 2014. Note that it will still only require a 90 day attestation linked to one of the calendar quarters for 2014. Reporting of clinical quality measures will depend on whether you are meeting 2013 or 2014 objectivesand measures
If you have upgraded to a 2014 CEHRT but are notable to “fully implement” all of the features youcan attest to 2014 Stage 1 objectives and measures.As stated in the proposed rule:“A provider’s ability to fully implement all of the functionality of2014 Edition CEHRT may be limited by the availability and timing ofproduct installation, deployment of new processes and workflows,and employee training. This effect is compounded for providers inStage 2 as some providers may not be able to fully implement all ofthe functions included in 2014 Edition CEHRT that are necessary tomeet the Stage 2 objectives and measures in time to complete theirEHR reporting period in 2014. Therefore, under our proposal,providers who are scheduled to begin Stage 2 for the 2014 EHRreporting period but are unable to fully implement all the functions oftheir 2014 Edition CEHRT required for Stage 2 objectives andmeasures due to delays in 2014 Edition CEHRT availability would havethe option of using 2014 Edition CEHRT to attest to the 2014 Stage 1objectives and measures for the 2014 EHR reporting period. Providerswho are scheduled to begin Stage 2 in 2014 who choose this optionmust attest that they are unable to fully implement 2014 EditionCEHRT because of issues related to 2014 Edition CEHRT availabilitydelays when they attest to the meaningful use objectives andmeasures.”
Reporting Period for 2014 (remainsunchanged even with proposedrule)
Stage 2 Requirements
Menu Objectives
Submit electronic syndromicsurveillance data to publichealth agencies
Record electronic notes inpatient records
Imaging results accessiblethrough CEHRT
Record patient family healthhistory
Report cancer cases to a publichealth central cancer registry
Report specific cases to aspecialized registry
Qualified Clinical Data Registry(QCDR)For 2014, these registries are CMSapproved entities that collects medicaland/or clinical data for the purpose ofpatient and disease tracking to fosterimprovement in the quality of care providedto patients.
U S Wound Registry (USWR)The U.S. Wound Registry (USWR) is one of many specialtyregistries developed and operated by the non-profit ChronicDisease Registry. It became a PQRS registry at the outset of theregistry process in 2008 and is one of most experienced PQRSregistries. As a non-profit organization focused on quality ofcare for patients with chronic wounds, the USWR began toshed light on “gaps in practice” such as why off-loading is sopoorly implemented for diabetic foot ulcers. 1 USWR workedwith the Institute for Clinical Outcomes Research (ICOR) todevelop the Wound Healing Index (WHI) to stratify patients byseverity in order to provide a more equitable way to reporthealing outcomes.2 In conjunction with the Alliance of WoundCare Stakeholders (AWCS) USWR submitted new wound carequality measures to CMS in 2009 and 2011 and started the “Dothe Right ThingTM” project to pilot test these quality measuresin outpatient wound centers (e.g. DFU off-loading, venous ulcercompression, vascular assessment of patients with leg ulcers).3For many years the USWR has lead the way in PQRS reportingfor wound care clinicians.
APMA relationship with USWR Complete the survey ng on March 31, 2014 when specifications are postedonline, select the APMA recommended measures and ask youEHR vendor to program the specifications into your EHR. Youmay want to have to contact your EHR vendor beforehand todiscuss any integration issues.Download the Business Associate Agreement (BAA) from theUSWR website (http://www.uswoundregistry.com), sign it,and return it by fax to the USWR at 832-550-2941 or toenrollment@uswoundregistry.com.Once your vendor has completed the programming to allowyou to submit your measures, you are ready to create anaccount for payment.Work with your assigned USWR representative to finish yourset up and begin reporting!APMA members receive a 50 discount
What If None of the MenuObjectives Are Relevant? It’s rare, but it’s possible that none of themenu objectives are applicable to your scopeof practice. If that is the case for you andyou qualify for all of the exclusions for eachof the menu objectives, then you can select 3menu objectives and claim the exclusion foreach.However, if you do not qualify for all of theexclusions to the menu objectives, you mustgo back and select menu objectives on whichyou can report.
Clinical Quality Measures (CQMs)
Changes to CQMs in 2014Beginning in 2014, the reporting of clinicalquality measures (CQMs) will change forall providers. EHR technology that has been certified tothe 2014 standards and capabilities willcontain new CQM criteria, and eligibleprofessionals will report using the new2014 criteria regardless of whether theyare participating in Stage 1 or Stage 2 ofthe EHR Incentive Programs.
How to Submit CQM Data in 2014 All Medicare eligible professionals have the option ofsubmitting three months of CQM data online throughthe CMS Registration & Attestation System. Medicare eligible professionals also have the option tosubmit a full year of data electronically using theQRDA format to receive credit for the EHR IncentiveProgram and the Physician Quality Reporting System. Please note that your attestation for the MedicareEHR Incentive Program is not complete until yousubmit clinical quality measure data, so your EHRincentive payment will be held until your electronicsubmission is processed.
How to Select CQMs in 2014Beginning in 2014, eligible professionals must select and report on 9 of apossible list of 64 approved CQMs for the EHR Incentive Programs. There is also a new requirement in 2014 that the quality measuresselected must cover at least 3 of the 6 available National Quality Strategy(NQS) domains, which represent the Department of Health and HumanServices’ NQS priorities for health care quality improvement. The 6domains are: Patient and Family EngagementPatient SafetyCare CoordinationPopulation and Public HealthEfficient Use of Health Care ResourcesClinical Processes/EffectivenessYou can find a complete list of the 2014 CQMs for the EHR IncentivePrograms and their associated National Quality Strategy domains at:Clinical Quality Measure webpage at tion/ EHRIncentivePrograms/ClinicalQualityMeasures.html
Potential CQMs for PodiatristsPreventive Care and Screening: Tobacco Use: Screening and Cessation Intervention(Population and Public Health)Preventive Care and Screening: Influenza Immunization (Population and Public Health)Pneumonia Vaccination Status for Older Adults (Clinical Processes/Effectiveness)Diabetes: Eye Exam (Clinical Processes/Effectiveness)Diabetes: Foot Exam (Clinical Processes/Effectiveness)Diabetes: Hemoglobin A1c Poor Control (Clinical Processes/Effectiveness)Hemoglobin A1c Test for Pediatric Patients (Clinical Processes/Effectiveness)Diabetes: Urine Protein Screening (Clinical Processes/Effectiveness)Diabetes: Low Density Lipoprotein (LDL) Management (Clinical Processes/Effectiveness)Falls: Screening for Future Fall Risk (Patient Safety)Documentation of Current Medications in the Medical Record (Patient Safety)Closing the referral loop: receipt of specialist report (Care Coordination)Preventive Care and Screening: Body Mass Index (BMI) Screening and Follow-Up (Populationand Public Health)Preventive Care and Screening: Screening for High Blood Pressure and Follow-Up Documented(Population and Public Health)
CQMs are a reporting requirementUnlike the Physician Quality ReportingSystem (PQRS), reporting CQMs forMeaningful Use is a reportingrequirement only—you do not have tomeet a threshold for performance. It is acceptable to report CQMs in whichyou have patients in the denominator butzero in the numerator (you did notperform the quality measure)
RESOURCES
QuestionsJames R. Christina, DPMjrchristina@apma.org301-581-9265
Stage 2 Requirements Menu Objectives Submit electronic syndromic surveillance data to public health agencies Record electronic notes in patient records Imaging results accessible through CEHRT Record patient family health history Report cancer cases to a public health central cancer registry Report specific cases to a specialized registry