Transcription
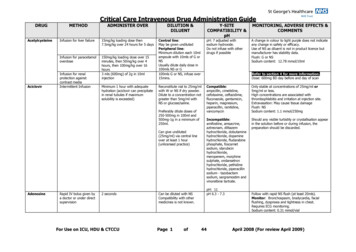
Critical Care Intravenous Drug Administration GuideDRUGAcetylcysteineAciclovirMETHODADMINISTER OVERDILUTION &DILUENTInfusion for liver failure15mg/kg loading dose then7.5mg/kg over 24 hours for 5 daysInfusion for paracetamoloverdose150mg/kg loading dose over 15minutes, then 50mg/kg over 4hours, then 100mg/kg over 16hours3 mls (600mg) of 2g in 10mlinjectionInfusion for renalprotection againstcontrast mediaIntermittent InfusionMinimum 1 hour with adequatehydration (aciclovir can precipitatein renal tubules if maximumsolubility is exceeded)Y-SITECOMPATIBILITY &pHMONITORING, ADVERSE EFFECTS &COMMENTSCentral line:May be given undilutedPeripheral line:Minimum dilution each 10mlampoule with 10mls of G orNSUsually dilute daily dose in100mls NS or G100mls G or NS, infuse over15mins.pH: 7 adjusted withsodium hydroxide.Do not infuse with otherdrugs if possibleA change in colour to light purple does not indicateany change in safety or efficacy.Use of NS as diluent is not in product licence butmanufacturer has stability data.Flush: G or NSSodium content: 12.78 mmol/10mlReconstitute vial to 25mg/mlwith W or NS if dry powder.Dilute to a concentration notgreater than 5mg/ml withNS or glucose/saline.Compatible:ampicillin, cimetidine,cefotaxime, ceftazidime,fluconazole, gentamicin,heparin, magnesium,piperacillin, ranitidine,vancomycinOnly stable at concentrations of 25mg/ml or5mg/ml or less.High concentrations are associated withthrombophlebitis and irritation at injection site.Extravasation: May cause tissue damageFlush: NSSodium content: 1.1 mmol/250mgIncompatible:amifostine, amsacrine,aztreonam, diltiazemhydrochloride, dobutaminehydrochloride, dopaminehydrochloride, fludarabinephosphate, foscarnetsodium, idarubicinhydrochloride,meropenem, morphinesulphate, ondansetronhydrochloride, pethidinehydrochloride, piperacillinsodium - tazobactamsodium, sargramostim andvinorelbine tartrate.Should any visible turbidity or crystallisation appearin the solution before or during infusion, thepreparation should be discarded.Preferably dilute doses of250-500mg in 100ml and500mg-1g in a minimum of250ml.Can give undiluted(25mg/ml) via central lineover at least 1 hour(unlicensed practice)AdenosineRapid IV bolus given bya doctor or under directsupervision2 secondsFor Use on ICU, HDU & CTCCUCan be diluted with NSCompatibility with othermedicines is not known.Page 1ofRefer to section 4 for more information.Dose: 600mg BD day before and day of scanpH: 11pH 6.3 - 7.344Follow with rapid NS flush (at least 20mls).Monitor: Bronchospasm, bradycardia, facialflushing, dyspnoea and tightness in chest.Requires ECG monitoring.Sodium content: 0.31 mmol/vialApril 2008 (For review April 2009)
Critical Care Intravenous Drug Administration nfusion viacentral line withpumpIV bolus undersupervision of adoctorInfusionAlbumin 20%InfusionAlfentanilContinuousInfusion withpumpIV bolus(under supervisionof a doctor in nonventilatedpatients)ADMINISTEROVERTitrate to responseDILUTION &DILUENTY-SITE COMPATIBILITY& pHGeneral ICU/HDUDilute 5mg in 50ml, 10 mgin 50ml or 15 mg in 50mlG (or NS)Cardiac ICUDilute 2mg, 4mg, 8mg or16mg in 50ml G (or NS)1mg in 10ml (1 in 10,000)MinijetNormal blood volume: 12ml/minHypovolaemia or shock:up to 1 L/hourPlasma exchange: up to30ml/minuteNormal blood volume: 12ml/minuteHypovolaemia or shock:up to 120ml/hourLoading dose 50-100micrograms/kg over 10mins or less followed byinfusion titrated to effectUndilutedDiluted 1mg/ml with G orNS or HRefer to Y-Site CompatibilityChartCompatible: atropine (in G only),doxapram.Incompatible: lignocaineStrengths above 15mg in 50ml should be reserved forexceptional circumstances or if requested by doctor.Less stable in NS. Infusions are given by central line,in emergency situations bolus doses may have to begiven by peripheral iv access.Monitor: BP, HR, intra-arterial or PCW catheter bloodpressure and cardiac monitoringpH: 2.5-3.6Extravasation: may cause tissue damageDo not flushDo not mix with any other drugs,infusions or blood productspH: 6.7-7.3Do not use if turbid or contains a depositRefer to Y-Site CompatibilityChartpH: 4.3-6Minimum 30 seconds inspontaneously breathingpatientsFor Use on ICU, HDU & CTCCUMONITORING, ADVERSE EFFECTS &COMMENTSMonitor: rarely allergic reaction. Use within 3 hoursof piercing container sealSodium content: 20% contains 50-120 mmol/L4.5% contains 100-160 mmol/LUse sedation score and pain tool to titrate to effectMonitor: BP, HR, RR, respiratory depression, apnoea,bradycardia, hypotension. Ensure maintenance ofventilation.Flush: NS or GPage 2of44April 2008 (For review April 2009)
Critical Care Intravenous Drug Administration GuideDRUGMETHODAlteplase(Recombinanthuman tissue-typeplasminogenactivator)IV bolusIntermittentinfusionAmikacinIV fusion with apumpADMINISTEROVERDILUTION &DILUENTY-SITE COMPATIBILITY& pHMONITORING, ADVERSE EFFECTS &COMMENTSAccelerated regimen inMI: give 15mg as a bolusover 2 minutes, followedby:Dilute each vial with waterfor injection provided. Ifnecessary further dilutewith NS to concentration of0.2mg/ml or above.Incompatible: Do not use W or Gfor dilution. Heparin, GTN,dobutamine, dopamine.Manufacturers recommend do notmix with any other drugsReconstituted vial stable for 24 hours in the fridge or 8hours at room temperature.50mg over 30minutes,and then 35mg over60minutes.Adjust dose in patients 65kg as per SPC2 - 3 minutes for dosesless than 500mg30-60 minutesLoading dose 5mg/kgover at least 20 minutesthen continuous infusionof 500micrograms/kg/hourMonitor: reperfusion arrhythmias, increased risk ofbleeding. May rarely cause allergic reactions.Flush: NSpH 7.3Can be diluted in 10 - 20mlNSCompatible: metronidazole,ranitidine, vancomycinDilute with 50-100ml NS,G.Preferred concentration2.5mg/ml in NS.Incompatible: amphoteracin,cephalosporins, erythromycin,penicillins, phenytoin, potassiumchloride, heparin, thiopentone,tetracyclines, vitamins B and C,nitrofuranoin, warfarinUsually diluted 500mg in250 to 500mls (1-2mg/ml)in NS or G.May use undiluted(25mg/ml) via central lineat rate not exceeding25mg/minute.pH: 4.5Refer to Y-Site CompatibilityChartCompatible: calcium gluconate,meropenem, metoclopramide.Serum level monitoring required. Usually givenonce daily at 12noon, dose 15-20mg/kg. Troughlevel taken at 6am should be less than 5 mcg/ml.Flush: NS, GSodium content: 0.72mmol/500mgSolution may darken from colourless to pale yellow butthis does not indicate loss of potency.Monitor: Serum theophylline level required(therapeutic range 10-20 mg/L). BP, HR, ECG,tachycardia and hypotension. Arrhythmias andconvulsions may occur if infusion rate is too fast.Extravasation: may cause tissue damageIncompatible: cefotaxime,ciprofloxacin, clindamycin,hydralazine, pethidine.Flush: NS, GpH: 8.8-10For Use on ICU, HDU & CTCCUPage 3of44April 2008 (For review April 2009)
Critical Care Intravenous Drug Administration GuideDRUGAmiodaroneAmoxicillinMETHODContinuous orIntermittentinfusion withpump preferablyby central lineIV bolus inmedicalemergency underdirect supervisionof a doctorIV bolusADMINISTEROVERDILUTION &DILUENTLoading dose (5mg/kg in250ml G) by intermittentinfusion over 20 to 120minutes, followed bycontinuous infusion (up to15mg/kg in up to 500mlG over 24 hours)Dilute in G only. Stabilityconcentration dependent do not dilute to less than300mg in 500mls. Usualdilution 300mg in 100mlsG, then 900mg-1.2g in250mls G (ICU/HDU) or600-900mg in 46-48ml Gas appropriate (run at2ml/hr)(Cardiac ICU).3 minutes300mg in 10ml minijet3-4 minReconstitute each 500mgwith 10ml W and each 1gvial with 20ml W.Y-SITE COMPATIBILITY& pHRefer to Y-Site CompatibilityChartCompatible: gentamicin,isoprenaline, lignocaine,metronidazole, vancomycinIncompatible: NS, sodiumbicarbonatepH: 3.5-4.5Compatible: few compatibilitiesknown, contact pharmacist foradviceIncompatible: ciprofloxacin,midazolam, aminoglycosides (e.g.amikacin, gentamicin)Displacement value:0.4ml per 500mg0.8ml per 1gMONITORING, ADVERSE EFFECTS &COMMENTSIrritant, use central line if possible, especially forrepeated or continuous infusions. Use via peripherallines may cause discomfort and inflammation.Monitor: BP, HR, RR, rapid administration may causehypotension & anaphylactic shock, sweating, nauseaand in patients with respiratory failure, bronchospasmand apnoea.Cardiac monitoring required.Extravasation: may cause tissue damageFlush: GSodium content: nilFlush: NSSodium content: 1.6 mmol/500mg; 3.3mmol/1gThrombophlebitis may occur at injection site.pH: 8.6-8.8IntermittentinfusionOver 30-60 minutesReconstitue as above. Addto 50-100ml of G or NSFUNGIZONE Calculation Example for 17.5mg dose (250 micrograms /kg for 70kg patient)Reconstitute as below in 500mls buffered glucose 5%Flush line with glucose 5%Infuse 1mg over 30 mins (i.e. 1/ 17.5 x 500 28mls over 30 mins – 60mls/hr)Stop infusion and monitor patient for 30 minsRestart infusion and run over 4 hour (125mls/hr)Flush line with glucose 5%AMBISOME Calculation Examplefor 50mg dose (1mg/kg for 50kg patient)Reconstitute as below in 250mls glucose 5% and protect from lightFlush line with glucose 5%Infuse 1mg over 10mins (1/50 x 250 5mls over 10 mins 30ml/hr for 10 minsStop infusion and monitor patient for 30 minsRestart infusion and run over 60 mins (250mls/hr)Flush line with 5% glucoseFor Use on ICU, HDU & CTCCUPage 4of44April 2008 (For review April 2009)
Critical Care Intravenous Drug Administration GuideDRUGMETHODAmphotericindeoxycholate(FUNGIZONE )IntermittentInfusionTest dose of 1mg over20-30minutesIntermittent infusion:2 - 4 hours.Decrease rate to decreaseincidence of side effectse.g. give over 6 hours.CHECK THAT YOUARE USING THECORRECTFORMULATION.Risk of confusionbetween:Fungizone andAmbisomeDILUTION &DILUENTY-SITE COMPATIBILITY& pHMONITORING, ADVERSE EFFECTS &COMMENTSReconstitute each vial with10ml W, then dilute dosewith G to which 1-2mls ofamphotericin buffer (SGHformula) has been addedbeforehand.Compatible: buffered G, (heparinor hydrocortisone sodium succinatein limited concentrations)A test dose is usually given at the start of a newcourse, by infusing 1mg in 10-50ml G over 20-30minutes and observing for signs of anaphylacticreaction for a minimum of 30 minutes. The companyadvise that patient responses to the test dose may notbe predictive of subsequent severe side effects.Dilution:Peripheral line: 100micrograms/mlIncompatible: NS,benzylpenicillin, calcium salts,dobutamine, dopamine, fluconazole,frusemide, gentamicin,ondansetron, piperacillin, Potassiumchloride, ranitidinepH: 5.7Central line: up to amaximum of 500micrograms/mlIntermittentInfusionNormal dose range:250microgramsg/kg, iftolerated increasingslowly to 1mg/kg daily;max. (severe infection)1.5mg/kg daily or onalternate daysTestdose of 1mg over 10minutesIntermittent infusion:30-60 minutesCHECK THAT YOUARE USING THECORRECTFORMULATION.Risk of confusionbetween:Fungizone andAmbisomeProtect from light.Normal dose range:1mg/kg daily, increasingslowly to 3mg/kg daily.Febrile neutropenia:testdose followed by3mg/kg dailySee above forcalculationexampleBuffer is added to keep pH of G above 4.2 to preventprecipitation.Monitor: arrhythmias, convulsions, anaphylaxis,fever, chills, rigors, headache, vomiting. Rapid infusionmay increase side effects. May cause pain andthrombophlebitis at injection site.Protect from light.See above E )ADMINISTEROVERFlush: G before and after administration or useseparate lineAn in-line filter (pore sizeno less than 1 micron) maybe used.Sodium content: 0.25mmol/50mgCompatible: G and WAdd 12ml W, andimmediately shakevigorously for at least 30seconds until completedispersion is obtained.Incompatible: NS. Do not infusewith any other drugs or infusionfluids.Monitor: Chills & rigors, anaphylaxis, headache,vomitingpH:5-6Resulting concentration4mg/ml. Calculate theamount of this solution tobe further diluted.Store in fridge (2-8 C).A test dose is usually given at the start of each newcourse of treatment, by infusing 1mg in 10ml G over10 minutes and observing for signs of anaphylacticreaction for at least 30 minutes.Use infusion within 6 hours.Flush: G before and after administration or useseparate line.Withdraw the requiredvolume of ambisome into asyringe.Sodium content: less than 0.5 mmol/vialAdd this volume to therequired amount of G viathe 5-micron filterprovided, to give a finalconcentration between 200micrograms to 2mg per ml(usually 250mls)Stored at room temperature.For Use on ICU, HDU & CTCCUPage 5of44April 2008 (For review April 2009)
Critical Care Intravenous Drug Administration GuideDRUGAprotininMETHODADMINISTEROVERDILUTION &DILUENTY-SITE COMPATIBILITY& pHMONITORING, ADVERSE EFFECTS &COMMENTSMonitor: First 1ml of initial dose should always beadministered at least 10 minutes prior to the remainderof the dose due to the risk of allergic reactions. Bestgiven by central line, peripheral administration maycause thrombophlebitis.IV bolusMaximum rate:5ml-10/minuteProvided ready diluted.Can be diluted with NSIncompatible: Do not infuse withany other drugs includingInfusion20-50ml/houror GheparinpH: 5-7Flush: NSSodium content: 7.7 mmol/50mlArgipressinAtenololSee vasopressinIV bolus undersupervision of adoctorIntermittentInfusion withpumpMaximum rate1mg/minuteDose: 2.5mg repeated at5 minute intervals to amaximum of 10mgCan be diluted with NS orGCompatible: morphine, pethidineDose: 150mcg/kg over 20minutes repeated every12 hours if requiredDilute to 50mls with NS, GpH: 6Excessive bradycardia can be countered with IVatropine 0.6-2.4mg given in 0.6mg doses.Overdose may be treated with IV glucagonMonitor: BP, HR, severe bradycardia andhypotension. Can cause conduction defects,monitor ECG. Rapid infusion increases incidence ofside effects.Flush: NS or GSodium content: 1.3-1.8 mmol/5mgFor Use on ICU, HDU & CTCCUPage 6of44April 2008 (For review April 2009)
Critical Care I
Critical Care Intravenous Drug Administration Guide For Use on ICU, HDU & CTCCU Page 1 of 44 April 2008 (For review April 2009) DRUG METHOD ADMINISTER OVER DILUTION & DILUENT Y-SITE COMPATIBILITY & pH MONITORING, ADVERSE EFFECTS & COMMENTS Acetylcysteine Infusion for liver failure 15mg/kg loading dose then 7.5mg/kg over 24 hours for 5 days pH: 7 adjusted with sodium











