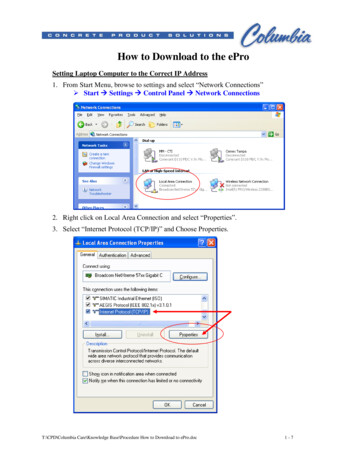
Transcription
EWPREVIEDC and ePRO MarketTrends and ServiceProvider PerformanceInfo@ISRreports.com 2013 Industry Standard Researchwww.ISRreports.com
act with confidenceReport OverviewThis report analyzes the current and future use of electronic data capture (EDC) and electronicpatient reported outcomes (ePRO) technologies in clinical trials. ISR quantifies study volumeand vendor preference, identifies operational improvements, develops implications andrecommendations for sponsors and service providers, and discusses the movement towards “eClinical”services. The report also provides extensive product profiles of 20 EDC and 14 ePRO products based on710 unique end-user experiences.101Site Coordinators /Principal Investigators155Pages167Major Sections:1.Executive Summary: In this section ISR summarizes the key findings and developsimplications and recommendations for sponsor and service provider organizations.2.EDC Market Adoption: Presents the current state of the EDC market including the numberof systems sites use at one time, their preference for EDC vs. paper, and the current andfuture EDC study penetration by development phase.3.EDC Operational Improvements: Outlines suggested improvements for EDC software andexamines the most important EDC attributes that can help improve study operations.4.EDC Service Provider Performance: Provides sponsors and service providers a look intothe most preferred EDC vendors and profiles how sites rate each of the 20 leading EDCservice providers on six key performance attributes.5.ePRO Market Adoption: Presents the current state of the ePRO market including thenumber of systems sites use at one time, their preference for ePRO vs. paper diaries, andthe current and future ePRO study penetration by development phase.6.ePRO Operational Improvements: Outlines suggested improvements for ePRO softwareand examines the most important ePRO attributes that can help improve study operations.7.ePRO Service Provider Performance: Provides sponsors and service providers a look intothe most preferred ePRO vendors and profiles how sites rate each of the 14 leading ePROservice providers on nine key performance attributes.8.eClinical Environment: Discusses site familiarity and desire for using direct data capturetechnologies, their use of eClinical solutions from the leading CROs, and identifies the bestCROs for eClinical support.Charts and GraphsQ2,2013Publication DateRegions Analyzed:Valuable for: Project Managers64% North America Outsourcing Clinical Operations28% Europe Data Management CROs8% Asia-Latin America-Middle East/ India Technology Service Providerswww.ISRreports.com 2013 Preview of: EDC and ePRO Market Trends and Service Provider Performance2
act with confidenceReport OverviewWhat you will learn in this report:How you can use this report:For Sponsors and CROs:For Sponsors and CROs: 710 Site-coordinators and PrincipalInvestigators’ product experiences andpreferences across 20 EDC and 14 ePROplatforms Select or uncover a new technology partnerthat enhances your relationships with sitesby understanding the needs, wants, andpreferences of clinical sites Past and future penetration of EDCand ePRO technologies (by Phase ofdevelopment) Learn how investigative sites wouldrecommend improving the trial process Which technology providers have thebest integration capabilities with othertechnologiesBenchmark your organization’s use of EDCand ePRO against broad industry averagesFor Technology Providers: Detailed performance profiles, productsatisfaction, and end-user preference for34 major technology providers across coreperformance attributes Current and future adoption estimates forEDC and ePRO technologiesFor Technology Provides: Benchmark your performance against yourcompetition Anticipate how changes in trial volume andadoption/penetration will affect your futurebusiness opportunities Identify operational improvements andproduct characteristics that impact trialsuccess and optimize user experienceEDC Products Included: Acceliant/ Trianz DSG OmniComm (TrialMaster) Bioclinica/ Phoenix Data Systems Medidata (Rave) OpenClinica Clinical Ink MedNet Solutions Oracle/ Phase Forward (InForm) Clinipace (Tempo) Medrio Registrat-MAPI Cmed (Timaeus) Merge Target Health DataLabs/ Perceptive Informatics Nextrials (Prism) Xclinical (Marvin) DATATRAK Octagon (Fuse)ePRO Products Included: Almac CRF Health Outcome Biomedical Systems(Michelangelo) DSG (eDiaryLink) Perceptive Informatics ERT/ invivodata PHT Bracket Global/ Arrowhead Exco Intouch Registrat-MAPI Clinipace (Tempo) ICOPhone (ICON) Symfowww.ISRreports.com 2013 Preview of: EDC and ePRO Market Trends and Service Provider Performance3
act with confidenceTable of ContentsCopyright and Usage GuidelinesClinipace (Tempo) profileIntroductionCmed (Timaeus) profileMethodologyDataLabs/ Perceptive Informatics profileRespondent Demographics and QualificationsDATATRAK profileMajor SectionsDSG profileExecutive SummaryMedidata (Rave) profileEDC Market AdoptionMedNet Solutions profileNumber of EDC systems currently usingMedrio profileEDC vs. paper CRF preferenceMerge profilePast two years: percent of trials using EDC bydevelopment phaseNextrials (Prism) profileNext two years: percent of trials using EDC bydevelopment phaseOmniComm (TrialMaster) profileSummary: percent of trials using EDC bydevelopment phaseOracle/ Phase Forward (InForm) profileEDC Operational ImprovementsMost impactful EDC product attributes for trialsuccessSuggested improvements to EDC systemsOctagon (Fuse) profileOpenClinica profileRegistrat-MAPI profileTarget Health profileXclinical (Marvin) profileePRO Market AdoptionMindset of sites heading into an EDC trialNumber of ePRO systems currently usingSolutions to reduce the time needed to conduct a trialePRO vs. paper diary preferenceEDC systems that best integrates with IVR systemsPast two years: percent of trials using ePRO vs. paperdiaries by development phaseEDC systems that best integrates with ePROtechnologiesEDC Service Provider PerformanceFamiliarity level of leading EDC providersUse/ experience level of leading EDC providersMost preferred EDC software applicationRationale for why an EDC system is preferredEDC vendor satisfactionHuman-machine interface (how intuitive the softwareis to operate)The process for dealing with queriesNext two years: percent of trials using ePRO vs. paperdiaries by development phaseSummary: percent of trials using ePRO vs. paperdiaries by development phasePast two years: percent of trials using a PROcomponentNext two years: percent of trials using a PROcomponentSummary: percent of trials using a PRO componentePRO Operational ImprovementsSpeed of the applications, how fast the pages loadMost impactful ePRO product attributes for trialsuccessTechnical support from the vendorSuggested improvements to ePRO systemsIntegration capabilities with other applications (ePRO,IVR)Mindset of sites heading into an ePRO trialOverall satisfaction with the EDC productAcceliant/ Trianz profileBioclinica/ Phoenix Data Systems profileClinical Ink profileSolutions to reduce the time needed to conduct a trialePRO systems that best integrates with IVR systemsePRO systems that best integrates with EDCtechnologiesePRO Service Provider Performancewww.ISRreports.com 2013 Preview of: EDC and ePRO Market Trends and Service Provider Performance4
act with confidenceTable of ContentsFamiliarity level of leading ePRO providersUse/ experience level of leading ePRO providersMost preferred ePRO software applicationRationale for why an ePRO system is preferredePRO vendor satisfactionHuman-machine interface (how intuitive the SW is tooperate) for the ePRO deviceMost beneficial eClinical features to improve siteoperationsMost used CROs in past 18 monthsMost recommended CROs for eClinical technologyBest CRO for eClinical supportRespondent DemographicsPractice settingHuman-machine interface (how intuitive the SW is tooperate) for the ePRO web reporting toolJob rolePatient/ subject satisfaction with the ePRO applicationClinical trial recent activityDurability of the products once the subject has thedeviceGeographyLogistic of getting the devices to your siteLogistic of returning the devices to the vendorTechnical support from the vendorIntegration capabilities with other applications (EDC,IVR)Overall satisfaction with the ePRO productClinical trial responsibilityFamiliarity with EDC and ePROPersonal number of clinical trials, past 12 monthsSite number of clinical trials, past 12 monthsIndustry/ clinical trial experienceTherapeutic area responsibilityAbout Industry Standard ResearchAlmac profileBiomedical Systems (Michelangelo) profileBracket Global/ Arrowhead profileClinipace (Tempo) profileCRF Health profileDSG (eDiaryLink) profileERT/ invivodata profileExco Intouch profileICOPhone (ICON) profileOutcome profilePerceptive Informatics profilePHT profileRegistrat-MAPI profileSymfo profileeClinical EnvironmentFamiliarity with EDC as source data – FDA guidanceInterest in direct data capture devicesExperience with integrated eClinical systemseClinical systems usedeClinical benefits to siteswww.ISRreports.com 2013 Preview of: EDC and ePRO Market Trends and Service Provider Performance5
act with confidenceSample PageSummary:Percent of trials using EDC by development PhaseSummary: percent of trials using EDC by development phase“Thinking back over the past two years, what percent of clinical trials conducted atyour site used EDC as the data collection tool (vs. paper CRFs)? Your best estimate isfine.”(Base 100)“Thinking ahead over the next two years, what percent of clinical trials conducted atyour site do you believe will use EDC as the data collection tool (vs. paper CRFs)? Yourbest estimate is fine.”(Base 68% 2013 Industry Standard Research84%PhaseIII74%Data available in full s.com 2013 E DC- ‐ePROM arketT rendsa ndS erviceP roviderP erformance2 5www.ISRreports.com 2013 Preview of: EDC and ePRO Market Trends and Service Provider Performance6
act with confidenceSample PageSamplesuggestedImprovementsimprovements (verbatim):EDCOperational “ALLOW SITES TO ENTER PRIOR CONCOMITANTMEDICATIONS ON SAME PAGE AS CURRENT CON MEDS“For each of the EDC product attributes below, please indicate how much impact each(HAVEIF MEDIS trialPRIORMEDhas on yourability CHECKBOXto successfully runa clinicalthat usesan OREDCCURRENTapplication.”(Base 100)MED), AND CAPTURE MEDICAL HISTORY EVENTS ON SAMEPAGE AS ADVERSE EVENTS (HAVE CHECKBOX IF adHISTORYOR ftwareMORE CLOSELY FOLLOWS CLINICAL PRACTICE PROCESSperformance.OF CAPTURING THIS INFORMATION. SPONSORS SHOULDNoimpactSlightimpactimpactHighimpactBE ABLE TOPARTIALOUTINFO ModerateIN PAGESFOR ANALYSIS;DON’T MAKE THE SITES DO IT FOR THEM BY ENTERINGHuman- ‐machineinterface(howintui[ve52%6%11%ON SEPARATEPAGES. 31%thesoewareistooperate)Most impactful EDC product attributes for trial success “THE AMOUNT OF UP FRONT TRAINING IS THE BIGGESTSpeedoftheapplica[ons,howfasttheHASSLE.” 5%6%pagesload49%40% “SOMETIMES THERE IS A QUESTION THAT WE ARE NOTSUREHOWIT SO WE NEED TO46%CHECK ECRF GUIDELINES — MAYBE IF THERE CAN BE A LINKTO ELECTRONIC GUIDELINE OR SOME KIND OF “HELP”38%WHAT THE14%BE ABLE 43%SIGNTHAT5%WE’LLTO READ DIRECTLYTechnicalsupportfromthevendorSPONSOR WANT. I THINK IT COULD BE ports.com 2013 E DC- ‐ePROM arketT rendsa ndS erviceP roviderP erformance2 6www.ISRreports.com 2013 Preview of: EDC and ePRO Market Trends and Service ProviderPerformance7
act with confidenceSample PageSamplePHT profileProduct Profile“For each of the EDC software vendors you indicated you have used in the past 18months, please indicate how satisfied you are with the following attributes.” (Base hedevicestoyoursiteSomewhatsa[sfied8%Human- rthe4%4%ePROdeviceHuman- on,where1 veryunsatisfied,2 somewhatunsatisfied,3 somewhatsatisfied,4 eLogis[cofreturningthedevicestothevendor3.08Human- rtheePROdevice3.08Human- 21.002.003.00www.isrreports.com 2013 E DC- ‐ePROM arketT rendsa ndS erviceP roviderP erformance1 334.00www.ISRreports.com 2013 Preview of: EDC and ePRO Market Trends and Service Provider Performance8
act with confidenceOrdering InformationTo obtain full access to this report, please select one of the following licenses:Single-user License A single-user license allows access to a single individual user. 4,900 USDSite-wide LicenseA site-wide license allows access to organization employeeswithin a particular geographic site/location (i.e. NYC orLondon office) 7,350 USDEnterprise-wideLicenseAn enterprise-wide license allows access to ALL employees inan organization – this is the recommended license if a reporthas wide spread relevance throughout an organization. 9,800 USDTo purchase the report with a credit card or invoice, simply click on the desired license aboveto be taken to the purchase form. If you’d like to inquire about a different payment method orhave questions, contact us at Sales@ISRreports.com or 1.919.301.0106.To schedule a call to discuss this report with one of our analysts, please e-mail us at info@ISRreports.com.About Industry Standard ResearchIndustry Standard Research (ISR) is the premier, full service market research provider to the pharmaand pharma services industries. With over a decade of experience in the industry, ISR delivers an unmatched level of domain expertise.For more information about our off-the-shelf intelligence and custom research offerings, please visitour Web site at www.ISRreports.com, email info@ISRreports.com, or follow us on twitter @ISRreports.www.ISRreports.com 2013 Preview of: EDC and ePRO Market Trends and Service Provider Performance9
the current and future ePRO study penetration by development phase. 6. ePRO Operational Improvements: Outlines suggested improvements for ePRO software and examines the most important ePRO attributes that can help improve study operations. 7. ePRO Service Provider Performance: Provides sponsors and service providers a look into