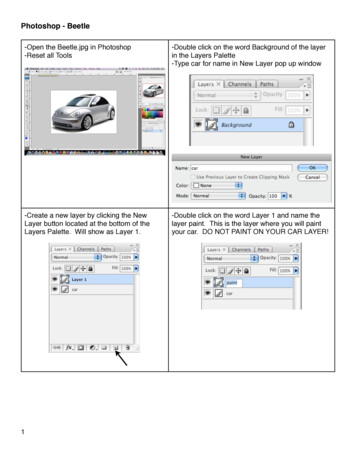
Transcription
Atomic Layer Deposition:Introduction to the Theory andCambridge Nanotech Savannah & FijiJ Provine & Michelle RinconNovember 1, 2012Stanford UniversityNNIN ALD Roadshow(special thanks to Ganesh Sundaram, Eric Deguns,Prof. H. Brongersma, and Prof. Fritz Prinz)
Methods for Depositing Thin FilmsMethodALDMBECVDSputterEvaporPLDThickness UniformitygoodfairgoodgoodfairfairFilm DensitygoodgoodgoodgoodfairgoodStep CoveragegoodvariesvariespoorpoorpoorInterface QualitygoodgoodvariespoorgoodvariesLow Temp. odgoodgoodvariesvariesgoodgoodgoodpoorDeposition RateIndustrial Applicability
What is ALD? aka How does it work? Originally called Atomic Layer Epitaxy– Has been around in one form or another foralmost 50 years A chemical vapor deposition method– However, the reaction is split into two parts– Each part is self-limiting and surface only– After completion of the two half-reactions thesystem is stable and a monolayer of film has beendeposited
ALD Example Cycle for Al2O3 DepositionTri-methylaluminumAl(CH3)3(g)Methyl group(CH3)AlCHHHHOSubstrate surface (e.g. Si)In air H2O vapor is adsorbed on most surfaces, forming a hydroxyl group.With silicon this forms: Si-O-H (s)After placing the substrate in the reactor, Trimethyl Aluminum (TMA) is pulsed intothe reaction chamber.
ALD Cycle for Al2O3Methane reactionproduct CH4HReaction ofTMA with OHHHCHHHHCCHHAlOSubstrate surface (e.g. Si)Trimethylaluminum (TMA) reacts with the adsorbed hydroxyl groups,producing methane as the reaction productAl(CH3)3 (g) : Si-O-H(s):Si-O-Al(CH3)2(s) CH4
ALD Cycle for Al2O3Methane reactionproduct CH4Excess TMAHHHCCHAlOSubstrate surface (e.g. Si)Trimethyl Aluminum (TMA) reacts with the adsorbed hydroxyl groups,until the surface is passivated. TMA does not react with itself, terminating thereaction to one layer. This causes the perfect uniformity of ALD.The excess TMA is pumped away with the methane reaction product.
ALD Cycle for Al2O3H 2OOHHHHHCHCAlOAfter the TMA and methane reaction product is pumped away,water vapor (H2O) is pulsed into the reaction chamber.
ALD Cycle for Al2O3Methane reaction productNew hydroxyl groupMethane reactionproductOxygen bridgesHOAlAlOAlOH2O reacts with the dangling methyl groups on the new surface forming aluminumoxygen (Al-O) bridges and hydroxyl surface groups, waiting for a new TMA pulse.Again metane is the reaction product.2 H2O (g) :Si-O-Al(CH3)2(s):Si-O-Al(OH)2(s) 2 CH4
ALD Cycle for Al2O3HOAlOAlOAlOThe reaction product methane is pumped away. Excess H2O vapor does not react withthe hydroxyl surface groups, again causing perfect passivation to one atomic layer.
ALD Cycle for Al2O3HHOOAlOHOAlOOAlOAlOAlOOOOOAlOOAlAlAlOOOOne TMA and one H2O vapor pulse form one cycle. Here three cycles are shown, withapproximately 1 Angstrom per cycle. Each cycle including pulsing and pumping takes e.g. 3 sec.Two reaction steps in each cycle:Al(CH3)3 (g) :Al-O-H(s)2 H2O (g) :O-Al(CH3)2(s):Al-O-Al(CH3)2(s) CH4:Al-O-Al(OH)2(s) 2 CH4
Signature Qualities of ALD Step One: Linear growth rate14001200y 1.259xThickness (Å)100080060040020000200400600800Number of Cycles10001200
Signature Qualities of ALD? Step Two: Self-limiting deposition/cycleSaturation Curve at 250 C1.61.4Growth Per Cycle1.21.00.80.60.40.20.00.00.51.01.5Precursor Dose (seconds)2.02.5
ALD “Window”Each ALD process has an ideal process “window” in which growth is saturated at amonolayer of film.Decomposition limitedCondensation limitedGrowth ivation energy limitedDesorption limited
ALD Window
Advantages of ALDUnique Chemistry Driven Process– Self-saturating reactions with surface– Thermal decomposition of precursor not-allowed– Low temperature and low stress(molecular self assembly)– Excellent adhesion Conformal Coating– Perfect 3D conformality: no line of sight issues– Ultra high aspect ratio ( 2,000:1)– Large area thickness uniformity and scalability Challenging Substrates– Gentle deposition process for sensitive substrates(i.e. biomaterials, plastics)– Coats challenging substrates (teflon, graphenegold)Growth Rate per Cycle600Film Thickness (Å) 50040030020010000200400600Number of CyclesTypical ALD processes have a growthrate between 0.5-1.5Å per cycle
Disadvantages of ALD Unique, Chemistry Driven Process– Not every material possible– Precursors can limit process due to reactivity / availability– Process limited by activation energy– No thermal decomposition of precursor allowed Conformal Coating– Deposition can be comparably slow: cycles times of 1 second to 1minute depending on substrate and temperature– Removal of excess precursor and by-products is required Challenging Substrates– Functionalization steps may be required
Let’s take an extra moment on conformality
ALD PrecursorsGood ALD precursors need to have the following characteristics:1.VolatilityVapor pressure ( 0.1Torr at T 200 C)Liquid at volatilization temperature without decomposition2.ReactivityAble to quickly react with substrate in a self-limiting fashion(most precursors are air-sensitive)3.StabilityThermal decomposition in the reactor or on the substrate is not allowed4.ByproductsShould not etch growing film and/or compete for surface sites5.Availability
ALD PrecursorsThe second precursor (Precursor B) must react with adsorbed monolayer (A) in order to formbonds and prepare the surface for another dose of (A)Some common precursors include:Oxidants – for oxidesWater (H2O), ozone, O2 plasma, alcohols (ROH), metal alkoxides [M(OR)x]ReductantsMetalsHydrogen gas (H2), ammonia (NH3),Silanes (Si2H6)NitridesAmmonia (NH3), N2 plasma,hydrazine (NH2-NH2)SulfidesHydrogen Sulfide (H2S)
Al2O3 From BeerCambridge NanoTech Experiment: replacement of H2O with beer
Al2O3 From BeerAl2O3 grown with H2O/TMAAl2O3 grown with beer/TMABoth 1000 cycles, 1000 Angstrom thick. The results are remarkably similar as thevapor draw allows the water/alcohol vapor to be distilled from the beer precursorcylinder, demonstrating the reduced requirement for high purity precursors if used incertain applications
Two deviations fromthe perfect ALD model Films are typicallyamorphous– Poly or crystalline can beachieved in properdeposition condition orwith annealHHOOAlOAlOAlOOOOAlOAlOAlOHOOOOAlOAlOAlO Nucleation– Not every self-limitinghalf-reaction fullyoccupies every availablesight every cycle– In fact, at some level thisis true for every filmdeposited– Substrate dependence– Chemistry dependence– Film roughness
A little more about nucleationPt from MeCpPtMe3 and O2250Thermal 270 C200Film Thickness (Å)Thermal 100 C1501005000200400600# of Cycles8001000
ALD ProcessesCambridge NanoTech has several standard recipes available as starting points for customers.However, there are many processes which have not been attempted in our applications lab.Many ALD processes exist:ALD Periodic TableElement included in at least one ALD materialElement not included in any ALD materialProf. Roy Gordon – Harvard UniversityJust because a “process” exists, it does not mean it is a “good process”
Oxides made by ALD
Pure Elements made by ALD
Nitrides made by ALD
Sulfides made by ALD
Carbides made by ALD
Fluorides made by ALD
Periodic table frequently updated athttp://www.cambridgenanotech.com/periodic
The SNF ALD nah
SNF ALD Websites
Background Summary Atomic Layer Deposition can deliver thin filmswith–––––Very precise thickness control ( Angstroms)Many materials (metals, dielectrics, magnetics )Highly conformalUniform over large areasEngineerable into “meta-materials” with alternatinglayers Downside:– Slow slow slow– Not all chemistries are created equal– Nucleation
Any Questions?
The Cambridge Nanotech Savannah
Main Components of an ALD SystemALD ManifoldChamberCarrier Gas Line(Vapor Draw)Main Vacuum ValveProcess Vacuum in 1001000mT rangeALD Pulse ValvesPrecursor Cylinders
Writing a Recipe A sequential list ofcommands Basic recipe– Length of precursorpulse– Length of purge cycle– Temperature of reactorand other components– # of cyclesStep command 0Cycles8flow5sccm013
Other recipe variables Stabilize– Select a heater and atemperature– The system waits toproceed until the heatermaintains that value fora few seconds– Allows consistentprocessing Stop-valve– Boolean command tocontrol the gate valve tothe pump– By temporarily valvingoff the pump you canleave the reactants inthe chamber longer– This can enhancecoverage of extremeaspect ratios (2000:1)
What you can tell from the pulse trainMissing pulsesindicate a problemMost likely theprecursor is out(should last 1000sof pulses beforerefilling/replacing)Height above base pressure should be 100mTorr for chamber saturation
Plasma Enabled (PE)ALDRemote Plasma as a reactant– Widens ALD window for materials by decreasing–––––activation energyAvoids precursor decomposition or damaging substrateswith limited thermal budgetRemote ICP source prevents substrate damage fromionsFaster deposition cycle timesFewer contaminates in filmsSmaller nucleation delayFilm Examples– Low temperature oxides– Metal nitrides– Metallic films High-Aspect Ratio Structures– Radical recombination prevents greater than 20:1Fiji PE-ALD chamber
Benefits of PlasmaDecreased nucleation for metallic films Pt from MeCpPtMe3 and O2400Precursor temp 90 CThermal 270 C350 Nucleation is eliminated whenusing O2 plasma as reactantConstant growth rate per cycleeven at low process tempThermal 100 C300Film Thickness (Å) Plasma 270 CPlasma 100 C2502001501005000200400600# of Cycles8001000
The Cambridge Nanotech Fiji
Main Components of an ALD SystemALD ManifoldCarrier Gas Line(Vapor Draw)Remote Plasma LineChamberProcess Vacuum in 1001000mT rangeALD Pulse ValvesPrecursor CylindersMain Vacuum Valve
Writing a Recipe A sequential list of commands Basic recipe––––––Length of precursor pulseLength of purge cycleTemperature of reactors etcProcess gas flowsPlasma power# of cycles
Other recipe variables Stabilize– Select a heater and atemperature– The system waits toproceed until the heatermaintains that value fora few seconds– Allows consistentprocessing Stop-valve– Boolean command tocontrol the gate valve tothe pump– By temporarily valvingoff the pump you canleave the reactants inthe chamber longer– This can enhancecoverage of extremeaspect ratios (2000:1)
What you can tell from the pulse trainMissing pulsesindicate a problemMost likely theprecursor is out(should last 1000sof pulses beforerefilling/replacing)Height above base pressure should be 100mTorr for chamber saturation
Final thoughts The field is rapidly changing––––More precursorsMore materialsMore variationsMore applications Like most Nanofabrication – Best chance for success is to get in the lab and pushexperiments Some resources:– www.cambridgenanotech.com– ition/ald/– pordeposition/ald/fiji-l– jprovine@stanford.edu; mmrincon@stanford.edu
Thank you.Any Questions?
Excess TMA Methane reaction product CH 4 H H C Trimethyl Aluminum (TMA) reacts with the adsorbed hydroxyl groups, until the surface is passivated. TMA does not react with itself, terminating the reaction to one layer. This causes the perfect uniformity of ALD. The excess TMA is pumped away with the methane reaction product. Substrate surface (e .