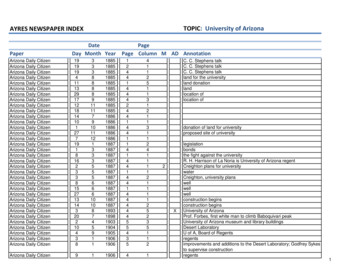
Transcription
Journal of the Arizona, New Mexico, Colorado and California ThoracicSocieties www.swjpcc.comMethylene Blue Treatment of Pediatric Patients in the Cardiovascular Intensive Care UnitAshley L. Scheffer, MD1,2Frederick A. Willyerd, MD1,2Allison L. Mruk, PharmD, BCPPS3Sarah Patel, BS2Lucia Mirea, MSc, PhD4Chasity Wellnitz, RN, BSN, MPH5Daniel Velez MD2,5Brigham C. Willis, MD, MEd2,6,71Division of Critical Care Medicine, Phoenix Children's Hospital, Phoenix, AZDepartment of Child Health, University of Arizona College of Medicine-Phoenix, Phoenix, AZ3Department of Pharmacy Services, Phoenix Children's Hospital, Phoenix, AZ4Department of Biostatistics, Phoenix Children’s Hospital, Phoenix, AZ5Division of Cardiovascular Surgery, Phoenix Children’s Hospital, Phoenix, AZ6Division of Cardiovascular Intensive Care, Phoenix Children’s Hospital, Phoenix, AZ7Department of Pediatrics, University of California Riverside School of Medicine, Riverside, CA2AbstractBackground: In both adults and children, hypotension related to a vasoplegic state has multipleetiologies, including septic shock, burn injury or cardiopulmonary bypass-induced vasoplegicsyndrome likely due to an increase in nitric oxide (NO) within the vasculature. Methylene blue isused at times to treat this condition, but its use in pediatric cardiac patients has not been describedpreviously in the literature.Objective: 1) Analyze the mean arterial blood pressures and vasoactive-inotropic scores ofpediatric patients whose hypotension was treated with methylene blue compared to hypotensivecontrols; 2) Describe the dose administered and the pathologies of hypotension cited formethylene blue use; 3) Compare the morbidity and mortality of pediatric patients treated withmethylene blue versus controls.Design: A retrospective chart review.Setting: Cardiac ICU in a quaternary care free-standing children’s hospital.Patients: Thirty-two patients with congenital heart disease who received methylene blue astreatment for hypotension, fifty patients with congenital heart disease identified as controls.Interventions: None.Southwest Journal of Pulmonary and Critical Care/2021/Volume 238
Measurements and Main Results: Demographic and vital sign data was collected for all pediatricpatients treated with methylene blue during a three-year study period. Mixed effects linearregression models analyzed mean arterial blood pressure trends for twelve hours post methyleneblue treatment and vasoactive-inotropic scores for twenty-four hours post treatment. Methyleneblue use correlated with an increase in mean arterial blood pressure of 10.8mm Hg over a twelvehour period (p 0.001). Mean arterial blood pressure trends of patients older than one year didnot differ significantly from controls (p 1.00), but patients less than or equal to one year of age hadincreasing mean arterial blood pressures that were significantly different from controls (p 0.02).Mixed effects linear regression modeling found a statistically significant decrease in vasoactiveinotropic scores over a twenty-four-hour period in the group treated with methylene blue (p 0.001). This difference remained significant comparted to controls (p 0.003). Survival estimatesdid not detect a difference between the two groups (p 0.39).Conclusion: Methylene blue may be associated with a decreased need for vasoactive-inotropicsupport and may correlate with an increase in mean arterial blood pressure in patients who are lessthan or equal to one year of age.Keywords: vasoplegic syndrome, methylene blue, systemic vascular resistance, vasodilation, congenital cardiacsurgery, congenital heart disease, nitric oxide, NO, hypotension, vasoplegia,IntroductionOne well recognized risk associated withplacing patients on cardiopulmonary bypass(CPB) during cardiac surgery is vasoplegicsyndrome (VS). VS is a constellation ofsymptoms comprised of hypotensionrefractory to volume resuscitation andinotropic support, an adequate to highcardiac output state, and low systemicvascular resistance (SVR) (1-4). In adultpatients placed on cardiopulmonary bypassthe incidence of VS is as high as 4.8%- 8.8%(1,2). For at risk adult populations, such asthose who have used heparin, angiotensinconverting enzyme inhibitors, or calciumchannel blockers pre-operatively, thisincidence increases to 44.4%-55.6% (3).Additionally, adult patients who experiencevasoplegia after cardiac surgery demonstratean increased mortality of 10.7%-24% (1,3).Since this syndrome does not respond toconventional fluid resuscitation andvasoactive therapy, patients who experiencevasoplegic syndrome often experience poorsystemic perfusion that can progress tomultisystem organ failure and ultimatelydeath (2).In both adults and children, hypotensionrelated to a vasoplegic state has multipleetiologies, including septic shock, burn injuryor cardiopulmonary bypass-inducedvasoplegic syndrome. Various studies havedemonstrated an increase in nitric oxide(NO) as the cause of this hypotension (4,6).Vascular endothelial and smooth muscle cellscontain enzymes that actively produce NO.Vasoplegia is hypothesized to result from thedisruption of blood vessel endothelialhomeostasis through increased inflammationand dysregulation of the nitric oxide andcyclic guanosine 3’, 5’ monophosphatepathway (cGMP) (5). Published literaturedemonstrates decreased morbidity andmortality when NO synthesis is inhibitedpreventing microcirculation impairment (4).Pharmacologic treatments that inhibit NOsynthase (NOS) have been developed in anattempt to decrease NO production indisease pathologies where the upregulation ofNO causes hypotension. Initial animal andhuman studies testing nonspecific NOSinhibitors showed NOS inhibition did reducehypotension and increase systemic vascularresistance (SVR) (8). However, nonspecificSouthwest Journal of Pulmonary and Critical Care/2021/Volume 239
NOS inhibition was also associated withsevere adverse side effects includingmyocardial depression with decreasedcardiac output, decreased oxygen delivery,and increased mortality, thereby making itunsafe for clinical treatment of vasoplegicsyndrome (8).In order for a pharmacologic agent tosuccessfully inhibit NO, while avoidingserious adverse events, it would theoreticallyneed to inhibit the NO pathway through adifferent mechanism. In cases of NOupregulation, methylene blue appears toinhibit soluble guanylate cyclase (sGC), adownstream biochemical messenger of NO,and ultimately decreases cGMP. cGMP is thefinal molecular messenger in the NOpathway. Theoretically, decreasing cGMPmight avoid the myocardial depression andother adverse side effects seen in nonspecificNO synthase inhibition. Methylene blue iscurrently approved by the United StatesFood and Drug Administration for thetreatment of methemoglobinemia, but hasbeen studied in the medical literature as anoff-label treatment for vasoplegic syndromein adults. Levin et. al. used methylene blue(MB) as a treatment of CPB-inducedvasoplegia in adults and showed a reductionin mortality in those who received thetreatment (1,6). In a study treating adults withnorepinephrine refractory VS Leyh et.al.demonstrated a subsequently higher SVRand decreased need for catecholaminetherapy in the methylene blue treatmentgroup (2,6).Whether methylene blue is an effectivetreatment for hypotension in pediatricpatients in the cardiovascular intensive careunit remains unknown. There is very limiteddata published on the use of methylene bluein pediatrics. Methylene blue is used,however, in pediatric cardiovascular intensivecare units to treat patients experiencing CPBinduced VS refractory to traditional clinicalmanagement based on the decreasedmortality reported in the adult literature.Pediatric patients represent a subpopulationwhose cardiac pathologies vary greatly fromthe adults examined in published studies.Due to the variability in cardiac pathology, weaim to describe the type of pathologies forwhich methylene blue was administered. Weexamine the association between methyleneblue and vital sign trends of pediatricpatients, specifically mean arterial bloodpressures and vasoactive-inotropic scores.Finally, we compare morbidity and mortalityof patients who received methylene bluetreatment to controls. In this way, our studyinvestigates if methylene blue is a safe andeffective treatment, in conjunction withconventional vasopressor therapy, forhypotension in a pediatric population withcongenital heart disease.Materials and MethodsThis retrospective chart review study wasapproved by the Institutional Review Boardat Phoenix Children’s Hospital and theInstitutional Review Board waived the needfor subjects to provide informed consent.Electronic medical records were queried toidentify patients who were treated withmethylene blue in the cardiac intensive careunit of a single, quaternary care free-standingchildren’s hospital from February 1st, 2013to June 30th, 2016. A clinically comparablecontrol sample not treated with methyleneblue from the same cardiac intensive careunit and time period was identified through apharmacy database. Control patients receivedtraditional medical therapy for vasoplegia,which included treatment with a combinationof epinephrine, vasopressin, and stress dosesteroids. Consistent with previous studies,methylene blue was dosed according toweight using a dose of 1-2mg/kg perinstitutional pharmacy recommendations.This study included any patient who receivedmethylene blue as treatment for hypotensionSouthwest Journal of Pulmonary and Critical Care/2021/Volume 2310
during the study period. Patients whoreceived methylene blue for a diagnostic orradiographic procedure instead of treatmentfor hypotension were excluded.For both treated and control patients, trainedinvestigators manually extracted demographicdata, vital sign data, and vasoactive-inotropicscores (VIS) during a designated collectionperiod. VIS composite scores reflecting theamount of inotrope and vasopressor supportrequired by infants postoperatively andinclude dopamine, dobutamine, epinephrine,milrinone, vasopressin, and norepinephrine.As methylene blue has a half-life of fivehours, mean arterial blood pressure (MAP)values were collected at the time themedication was administered and at 2, 4, 6,8, 10, and 12 hours post treatment, morethan two half-lives of the drug. Similarly, VISwere collected at the time of treatment and at6, 12, 18, and 24 hours post treatment, morethan four half-lives of methylene blue. Thecontrol cohort had similar electronic medicalrecord data collected for assessment.Morbidity and mortality data for both groupswas obtained from the Society of ThoracicSurgeons Database. Time-to-death in dayswas computed from the date of surgery to thedate of death from all causes.The distributions of demographic data,baseline clinical factors, cardiac surgicalrepair, and post-operative conditions weresummarized using descriptive statistics forboth the methylene blue and control group.Comparison between groups was performedusing parametric (Pearson Chi-square test, Ttest) or non-parametric (Fisher exact,Wilcoxon rank sum) analyses as appropriatefor the data distribution. Similar analysiscompared the amount of fluid resuscitationand steroid treatment between patients in themethylene blue group and the control group.Univariate mixed effect models were used toestimate the change in MAP and VIS overtime while controlling for extracorporealmembrane oxygenation (ECMO) support.Post-operative ventilator support, postoperative complications, length of stay, andmortality were described and comparedbetween the two groups using appropriatestatistical tests as listed above. Overall survivalwas displayed for each group using KaplanMeier curves and compared between the twogroups using the Log-rank test. All statisticaltests were 2-sided with significance evaluatedat the 5% level. Analyses were performedusing the statistical package SAS (SASInstitute 2011) and STATA (7).ResultsDuring the study period, methylene blue wasadministered on thirty-nine occasions to treatthirty-two unique patients. After excludingfour patients treated with methylene blue fordiagnostic procedures instead ofhypotension, the final sample treated withmethylene blue included twenty-eight uniquepatients, of which seven patients were treatedtwice, resulting in a total of thirty-fivemethylene blue treatments. Repeattreatments in the same patients were treatedas independent events as they were duringseparate clinical encounters. Indications forusing methylene blue included hypotensionsecondary to cardiogenic shock in sevenpatients (25%), post cardiopulmonary bypassvasoplegia in sixteen patients (57%), ECMOdecannulation hemodynamic instability intwo patients (7%), and septic shock in threepatients (11%) (Supplemental Digital Content1). Doses of methylene blue ranged from0.3mg/kg- 2mg/kg with an average dose of1.1mg/kg for the treatment cohort.Among patients less than one year of age,those treated with methylene blue receivedsurgery at a significantly younger age and hada lower mean weight at the time of surgerythan did controls (Table 1).Southwest Journal of Pulmonary and Critical Care/2021/Volume 2311
Table 1. Baseline characteristics for patientstreated with methylene blue and controls.SD standard deviation1P-value from Fisher exact test for categoricalvariables or Kruskal-Wallis test forcontinuous measures.Congenital heart disease diagnosis wascomparable between the two groups, exceptfor tetralogy of Fallot with zero patients (0%)among the methylene blue group, but tenpatients (21%) in the control group (Table 1).No significant differences were detected indisease severity as measured by the Society ofThoracic Surgeons (STAT) Category.At baseline mean arterial blood pressures(mean SD) were significantly lower (T-testp-value 0.004) in patients treated withmethylene blue (45mmHg 10) compared tocontrols (52mmHg 10). The averageincrease in mean arterial blood pressurefrom baseline to twelve hours did not varysignificantly (T-test p-value 0.40) betweenmethylene blue patients (8.5mmHg 13) andcontrols (5.6mmHg 16). However, whenanalyses were restricted to subjects less thanone year of age, a larger increase in meanarterial blood pressure was suggested (T-testp-value 0.08) for MB patients (8.5 14)compared to controls (1.4 16). Mixedeffects linear models examining MAPmeasurements over time among patients 1year with adjustment for ECMO, confirmeda significant increase in MAP over time forthose who were treated with MB (slopecoefficient 0.57, p-value 0.001) whereasno trend in MAP values was detected forcontrol patients 1 year (slope coefficient 0.08, p-value 0.6). Among patients 1 year,MAP increased over time for both MB andcontrols, with no detectable differencebetween the slopes estimates (Table 2).Table 2. Mixed effects linear regressionanalyses examining time trends in meanarterial pressure (MAP) and vasoactiveinotropic score (VIS) of patients treated withmethylene blue and controls by age.MAP mean arterial pressure; VIS vasoactive-inotropic scores; SE standarderror*All models included a random patient-levelintercept, assumed unstructured correlation,and were adjusted for ECMO.Figures 1A and 1B show the MAPmeasurements over time, and the estimatedslopes for MB and control patients adjustedfor clustering and ECMO.Southwest Journal of Pulmonary and Critical Care/2021/Volume 2312
Figure 1. Mean arterial blood pressure mixedeffects linear regression models stratified byage.The mean VIS at baseline was significantlyhigher in MB (27 26) compared to control(12 11) patients (T-test p-value 0.002).From baseline to 24 hours, MB patients hada significantly larger mean decrease in VISthan controls overall (T-test p-value 0.006).Analyses stratified by age detected asignificant negative trend in VIS for MBpatients, especially among MB patients 1year (Table 2). Weak negative trends in VISwere detected among controls (Figures 2Aand 2B).Figure 2. Vasoactive inotropic score mixedeffects linear regression models stratified byage.Patients treated with methylene blue wereextubated approximately twenty-four hourssooner than those in the control group(Table 3).Table 3. Outcomes among patients treatedwith methylene blue and controls.Southwest Journal of Pulmonary and Critical Care/2021/Volume 2313
SD standard deviation1P-value from Fisher exact test for categoricalvariables or Kruskal-Wallis test forcontinuous measuresHowever, methylene blue patients had higherincidence of ECMO support andmultisystem organ failure, but a lowerincidence of cardiac arrest compared tocontrols (Table 3). There were no reportedadverse effects from methylene blue use.Mortality at thirty days post operatively didnot vary significantly between groups (Table3). At discharge, methylene blue patients hadnotably higher mortality compared tocontrols (31% vs. 14%), but statisticalsignificance was not reached (Table 3). Therewas no difference in length of ICU stay orhospital length of stay between the twogroups (Table 3). Furthermore, no significantdifferences in survival were detected betweenthe methylene blue patients and controlpatients (Figure 3; Log-rank p-value 0.39);however, our study was not poweredadequately to show equivalence of a clinicaloutcome.Figure 3. Kaplan-Meier survival estimates forpatients treated with methylene blue versuscontrols.DiscussionOverall, we found that methylene blue usewas associated with a decreased need forvasoactive-inotropic support when comparedto the control cohort and may correlate withan increase in mean arterial blood pressureover time, specifically in those patients whoare less than or equal to one year of age.Vasoplegia results in increased mortalitybecause it often remains resistant to standardclinical interventions such as administrationof intravenous fluids and the use of multipleinotropic medications leading to refractoryshock and poor oxygen delivery in patientswho experience it (2). If a patient’s shockstate is unable to be reversed, vasoplegicsyndrome (VS) could lead to increasedmortality in vulnerable populations such aspediatric patients undergoingcardiopulmonary bypass for cardiac surgery.In our study, we demonstrated thatmethylene blue use was associated with anincrease in mean arterial blood pressure overa twelve-hour period and a decrease invasoactive-inotropic scores over a twentyfour-hour period. When compared withcontrols, the decrease in vasoactive-inotropicscore maintained statistical significance in allages, but mean arterial blood pressure trendswere only significant compared to controls inchildren less than or equal to one year of age.Southwest Journal of Pulmonary and Critical Care/2021/Volume 2314
These results support the theory thatmethylene blue could be an effectivetreatment for vasoplegia in the pediatricpopulation, although more prospectivestudies would be needed to verify causation.However, as mentioned above, given theretrospective nature of our study, thedifficulty in identifying a more completelymatched control cohort (especially for thegroup of patients 1 year of age), and thelimited numbers, such conclusions must betempered until such trials are performed.pressure vary greatly between ages, which canmake statistical interpretation of these vitalsign trends difficult. In our study,heterogeneity of age resulted in variability ofmean arterial blood pressure data that limitedour interpretation of vital signs trends unlessage groups were stratified. Ideally, we wouldhave examined all vital sign trends stratifiedby age to improve the accuracy of ourinterpretation. However, our population wastoo small to appropriately power such asubgroup analysis.During our evaluation we noted that theincrease in mean arterial blood pressure wasonly statistically significant when ages werestratified. In children older than a year, theincreasing mean arterial blood pressuretrends observed over time may have resultedfrom improvement of low cardiac outputsyndrome after cardiopulmonary bypasssince both the control and treatment cohortmixed effects linear regression models hadsimilarly increasing slopes that were notstatistically different from each other. In agesless than or equal to one year, however, thecontrol cohort mixed effects linear regressionmodel did not show any trend towardincreasing mean arterial blood pressures.Additionally, the methylene blue cohort hadan initial lower average mean arterial bloodpressure and a statistically significant trend upin mean arterial pressures over a twelve-hourperiod. Although this subgroup analysis was asmaller sample, the difference in the tworegression models suggests that there may bea correlation between the use of methyleneblue and increasing mean arterial bloodpressures in children less than or equal toone year of age.Attempting to identify the control groupwithout introducing bias may also havecontributed to the difference seen in meanarterial blood pressure trends between themethylene blue cohort and the controlcohort. There are multiple factors thatcontrol mean arterial blood pressure andvasoactive-inotropic scores. In an attempt tolimit cofounding factors, a control group wasselected using a pharmacy database thatidentified patients who received bothvasoactive-inotropic treatment and stress dosesteroids to treat refractory hypotension aftercardiac surgery to find a clinically comparablecohort. The control cohort varied slightly indemographic characteristics, but did notappear statistically different in fluidresuscitation or steroid use (SupplementalDigital Content 2). However, this remains asignificant limitation of the current study,given its small numbers, heterogeneouspopulation, and difficulty identifying a bettermatched control group. In the future, aprospective, randomized trial of methyleneblue in this population could address this.Both our treatment cohort and our controlcohort were very heterogeneous in certaindemographic characteristics, specifically inage and weight, but are very typical of theclinical patient population. Normal values forvital signs such as mean arterial bloodFor adult patients who experiencedvasoplegic syndrome, multiple studies havedemonstrated an overall reduction inmortality in patients who were treated withmethylene blue (1,2,6). However, unlike theadult studies, our study did not find anystatistically significant survival differencebetween the methylene blue cohort and theSouthwest Journal of Pulmonary and Critical Care/2021/Volume 2315
control cohort. Our study did demonstrate,however, that methylene blue was notassociated with increased mortality. Patientstreated with methylene blue were alsoextubated sooner that patients in the controlcohort. Speculatively, methylene bluetreatment may have been associated with lesscardiopulmonary liability, increasing theclinician’s confidence to wean towardextubation sooner than the control group. Inaddition, our study showed a higherincidence of extracorporeal membraneoxygenation support and multisystem organfailure in the methylene blue group ascompared to controls. This is likely a resultof the high incidence of refractoryhypotension and severe shock that led to theuse of methylene blue. There was nodifference between the two groups in theirnumber of intensive care days or hospitallength of stay. No adverse side effects directlyattributable to methylene blue were reportedin any of our cases, indicating it is apotentially safe treatment for vasoplegicsyndrome.cardiopulmonary bypass vasoplegicsyndrome would have resulted in a samplesize too small to appropriately power ourstudy.The definition of vasoplegia requires patientsto maintain a high cardiac output state. Therewere no objective measurements of cardiacoutput that could be identifiedretrospectively, thus our study relied onclinician estimation of high cardiac output. Innearly thirty percent of the methylene bluecohort, methylene blue was used as treatmentfor hypotension that was related to lowcardiac output or cardiogenic shock, notvasoplegia. The adult studies that showed adifference in mean arterial blood pressures aswell as mortality of patients were examiningmethylene blue treatment of hypotensionsecondary to vasoplegic syndromespecifically. Additional prospective studies inpediatric patients are needed to evaluate theeffectiveness of methylene blue in treatingvasoplegic syndrome.ConclusionOur study was designed as a retrospectivechart review and therefore had limitationsinherent with this design. We examinedblood pressure trends of any pediatric patientthat was given methylene blue forhypotension, regardless of thepathophysiology. Accurately pinpointing thejustification for methylene blue treatmentretrospectively was difficult especially giventhe complex nature of the patients’ diseaseprocesses, resulting in multiple reasons forhypotension cited in the electronic medicalrecord. We could not accurately limit ourpatient selection to patients withcardiopulmonary bypass-induced vasoplegiawithout introducing selection bias andtherefore decided to look at all patients whowere treated with methylene blue during thestudy period. Furthermore, limiting oursample size to only those patients whoreceived methylene blue as treatment for postMethylene blue may be a safe and effectivetreatment for vasoplegia in pediatric patientswith congenital heart disease. Methyleneblue use was associated with a decreasedneed for vasoactive-inotropic support whencompared to the control cohort and maycorrelate with an increase in mean arterialblood pressure over time, specifically in thosepatients who are less than or equal to oneyear of age. There was a statisticallysignificant decrease in ventilator daysbetween the methylene blue cohort and thecontrol cohort. There was no difference insurvival estimates between those patients whoreceived methylene blue versus controls.References1. Levin RL, Degrange MA, Bruno GF, DelMazo CD, Taborda DJ, Griotti JJ,Southwest Journal of Pulmonary and Critical Care/2021/Volume 2316
2.3.4.5.6.Boullon FJ. Methylene blue reducesmortality and morbidity in vasoplegicpatients after cardiac surgery. AnnThorac Surg. 2004 Feb;77(2):496-9.[CrossRef] [PubMed]Leyh RG, Kofidis T, Strüber M, FischerS, Knobloch K, Wachsmann B, Hagl C,Simon AR, Haverich A. Methylene blue:the drug of choice for catecholaminerefractory vasoplegia aftercardiopulmonary bypass? J ThoracCardiovasc Surg. 2003 Jun;125(6):142631. [CrossRef] [PubMed]Ozal E, Kuralay E, Yildirim V, Kilic S,Bolcal C, Kücükarslan N, Günay C,Demirkilic U, Tatar H. Preoperativemethylene blue administration in patientsat high risk for vasoplegic syndromeduring cardiac surgery. Ann Thorac Surg.2005 May;79(5):1615-9. [CrossRef][PubMed]Evora PR, Alves Junior L, Ferreira CA,Menardi AC, Bassetto S, Rodrigues AJ,Scorzoni Filho A, Vicente WV. Twentyyears of vasoplegic syndrome treatmentin heart surgery. Methylene blue revised.Rev Bras Cir Cardiovasc. 2015 JanMar;30(1):84-92. [CrossRef] [PubMed]Werner I, Guo F, Bogert NV, Stock UA,Meybohm P, Moritz A, Beiras-FernandezA. Methylene blue modulatestransendothelial migration of peripheralblood cells. PLoS One. 2013 Dec10;8(12):e82214. [CrossRef] [PubMed]Omar S, Zedan A, Nugent K. Cardiacvasoplegia syndrome: pathophysiology,risk factors and treatment. Am J Med Sci.2015 Jan;349(1):80-8. [CrossRef][PubMed]7. SAS Institute Inc. 2011. Base SAS 9.3Procedures Guide. Cary, NC: SASInstitute Inc.8. Farina Junior JA, Celotto AC, da SilvaMF, Evora PR. Guanylate cyclaseinhibition by methylene blue as an optionin the treatment of vasoplegia after asevere burn. A medical hypothesis. MedSci Monit. 2012 May;18(5):HY13-7.[CrossRef] [PubMed]9. Víteček J, Lojek A, Valacchi G, KubalaL. Arginine-based inhibitors of nitricoxide synthase: therapeutic potential andchallenges. Mediators Inflamm.2012;2012:318087. [CrossRef] [PubMed]10. Rutledge C, Brown B, Benner K,Prabhakaran P, Hayes L. A Novel Use ofMethylene Blue in the Pediatric ICU.Pediatrics. 2015 Oct;136(4):e1030-4.[CrossRef] [PubMed]11. Corral-Velez V, Lopez-Delgado JC,Betancur-Zambrano NL, Lopez-Suñe N,Rojas-Lora M, Torrado H, Ballus J. Theinflammatory response in cardiac surgery:an overview of the pathophysiology andclinical implications. Inflamm AllergyDrug Targets. 2015;13(6):367-70.[CrossRef] [PubMed]Presented, in part, in abstract form at the2018 Society of Critical Care MedicineConference in February 25-28, 2018, SanAntonio, TX.The authors have disclosed that they do nothave any potential conflicts of interest.Southwest Journal of Pulmonary and Critical Care/2021/Volume 2317
Journal of the Arizona, New Mexico, Colorado and California Thoracic . . 1