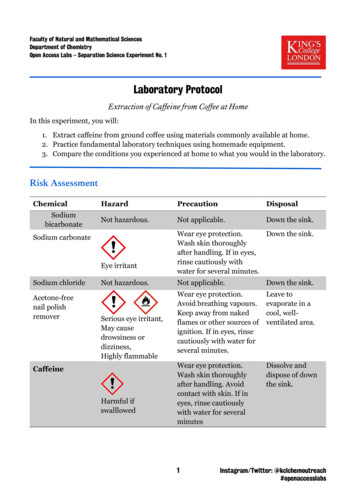
Transcription
Separation by Chromatography MethodsAnalytical Biochemistry3.1 Principle of Separation techniques3.2 Methods Based on Polarity (3.2.1-3.2.3)Biochemistry and Molecular Biology11.5 Partition Chromatography11.6 Ion Exchange Chromatography11.7 Gel Filtration Chromatography11.8 Affinity Chromatographyhttp://www.waters.com1
How Does Chromatography Work?Chromatography is a method forseparating the components of amixture by differential adsorptionbetween a stationary phase and amobile (moving) phaseMobile Phase ographyStationary Phase(固定相)Adsorptionchromatography2
Principles of Separation TechniquesAB 3.1MolecularCharacteristicPhysical propertySeparation as-liquid chromatographyLiquid-liquid chromatographyLiquid-solid chromatographyIonicChargeIon-exchange chromatographyElectrophoresisSize (mass)DiffusionShapeSedimentationLiquid bindingGel nAffinity chromatography3
Factors Involved in SeparationImpellingForce Gravitational (Ultracentrifugation) Electrokinetic (Electrophoresis) Hydrodynamic (Chromatography, 沖堤液驅動力)Retarding Force Single phase technique Molecular friction Electrostatic Dual phase technique Adsorption Binding Ionic interaction4
Three Major Methods in Chromatography Zonal (區帶)A band (zone) in a solvent system-- Change in pH, size etc DisplacementA band (zone) in two-phase solventsystem---Different affinity for the solidsupport (stationary/mobile phase) Frontal (前端)Large sample containing in mobilephase5
POLARITY (affinity of like moleculesfor each other)PARTITION BETWEEN TWO PHASESLiquid/LiquidSolid/LiquidLiquid/VapourA MAJOR FACTOR IN SEPARATION ISAdsorptionSolubilityAND THE METHODS INVOLVESolid adsorbentsTwo immiscibleliquidsA solution andIts vapourTHE METHODS ARE GENERALLY KNOWN graphy6chromatography
Different Kinds of Chromatography(characterized by the mobile phase) Liquid chromatography (includes columnchromatography, thin-layer, and HPLC)– Stationary phase: silica, alumina, etc.– Mobile phase (moving phase): organic solvents– Important properties: polarity Gas chromatography– Stationary phase: a film of a polymer or a wax. Thefilm must have a high boiling point– Mobile phase: gas (Helium is the usual carrier gas)– Important properties: boiling point7
Modes of Chromatography(characterized by shape of stationary phase Column chromatography Stationary phase is packed into a column Thin-Layer chromatography Stationary phase is coated onto glass, metallic or plasticplate.8
Liquid-Solid Chromatography (Adsorption)AB 3.2.1Adsorption (吸附):Some substances physically bind to thesurface of a solid polar substances Polar compound Large surface for adsorption Often by OH (hydroxy group) to form H-bonding9
Polarity of Selected Solutes and StrengthHydrocarbonHalogen eMethanol0.010.320.40.550.710.95Increasing PolarityAdsorption Energythe affinity of a solute with an adsorbent ( vary with adsorbant)Solvent Strengththe affinity of a solvent with an adsorbent ( vary with adsorbant)10
Stationary Phase: AluminaOOHOHOHOHAlAlAlAlAlOOOOOOAcidic: -Al-OHNeutral: -Al-OH -Al-OBasic: -Al-O11
Examples of Absorbents and ApplicationsAdsorbentStrengthApplicationSilicic acid(silica gel)StrongSteroids,amino uminium oxideStrongSteroids,esters,alkaloidsMagnesium carbonateMediumPorphyrinsCalcium akProteins12
Thin-layer chromatography and columnchromatography are different types ofliquid chromatography. The principle ofoperation is the same! The mobile (moving) phase is a liquid. The stationary phase is usually silica oralumina.---Æ a very polar layer ofadsorbent on an inert, flat support.13
Thin Layer Chromatography (薄層層析法)1. The surface of the plate consists of a very thin layer ofsilica on a plastic or aluminum backing. The silica is verypolar the stationary phase.2. Spot the material at the origin (bottom) of the TLC plate.3. Place the plate into a glass jar with a small amount of asolvent in the glass jar. the moving phase.4. Remove the plate from the bottle when the solvent isclose to the top of the plate.5. Visualize the spots (Ultraviolet light, color reagent etc)Non-polar compounds will be less strongly attracted to theplate and will spend more time in the moving phase. Thiscompound will move faster and will appear closer to thetop of the plate.Polar compounds will be more strongly attracted to the plateand will spend less time in the moving phase and appearlower on the plate.14
Thin-Layer Chromatography:A Two-Component Mixturesolvent frontcomponent BLess polar!component AMore polar!solvent frontcomponent Bcomponent Aoriginsolvent frontoriginoriginmixtureIncreasing Development Time15
Determination of Rf Values (Rate of Flow)A measure of the movement of a compoundcompared with the movement of solventsolvent frontRf of component A component BdAdSdSdBRf of component B component AdBdSdAorigin1. Only reported to two decimal places16
Thin-Layer Chromatography:Qualitative AnalysisIdeally, the Rf value should be thesame of a given compound usingthe same solvent(Practically, the movement dependson the structure and thickness ofthe layer, the amount of waterremaining and effect of thebinding agents.AB unknownAdvantages Simple Rapid Cheap17
Example: Thin-Layer ChromatographyOFluoreneFluorenoneOHFluorenola) Which one of these compounds is the least polar?b) Which one of these compounds is the most polar?c) What would be the relative order of separation onthe TLC plate remembering that CH2Cl2 is not verypolar?18
Liquid-Liquid ChromatographyPartition of a solute between two immiscible liquid phasesExample:High Performance Liquid Chromatography Partition chromatographyAdsorption chromatographyGel filtration chromatographyAffinity chromatographyIon-exchange chromatography19
High Performance Liquid ChromatographySmall and regularsupport media withstationary phaseProvide steady solventflow rate for isocratic orgradient mobile phaseAB 3.2.220
HPLC ColumnMost HPLC packings are porous.Most of the stationary phase surfacearea is on the inside of the particlesporous.A layer of alkyl chains bonded to thesilica surfaceThe composition of themobile phase provides thechemical environment forthe interaction of thesolutes with the stationaryphase.21
Chromatographic SeparationIn a liquid chromatographic process a liquid permeatesthrough a porous solid stationary phase and elutes thesolutes into a flow-through detector22
Sample Injector (AutoSampler)Full Loop Injection (cont’d)LOAD position6-portvalveCut-off the dilutedfrontSampleLOADFill 3 x loop volumeColumn(e.g. 3 x 5 µl)IN the LOAD position, the loop can befilled with the sample using a simplesyringe or an autosampler23
Sample Injector (AutoSampler)Full Loop Injection (cont’d)6-portvalveINJECT positionSampleINJECTColumnWhen the valve is turnedto INJECT position, thesample is washed into thecolumn.24
Detection Methods UV – Ultraviolet light--- most popular––––LampGrating/Lens - Wave length 190-350 nmFlowCellPhotoDiode - Differential Light Output RI – Refractive Index––––Universal analyte detectorSolvent must remain the same throughout separationVERY temperature sensitiveSometimes difficult to stabilize baseline FD – Fluorescence-greater sensitivity, not so popular– Excitation wavelength generates fluorescence emission at a higher wavelength– Analytes must have fluorophore group---not very common– Very sensitive and selective MS – Mass Spectrometry– Mass to charge ratio (m/z)– Allows specific compound ID25
HPLC Diode Array Detection Analysis -Absorbance ismeasured at two ormore wavelengthsElution TimepyrithionesAbsorption WavelengthPyrithione is an anti-oxidant26
Column Chromatographic SeparationStage 18 76 54 32 1287654 32 138 76 54 34875 6Addition ofMobile phaseAssumePartitioncoefficient 14 32 12StationaryPhase1MobilePhase27
Band BroadeningStage18 76 54 32 1287654 32 138 76 54 34ChromatographicPeak875 64 32 121GaussianDistribution28
Separation Efficiency: Plate TheoryThe plate theory suppose that the chromatographiccolumn contains a large number of separate layers, calledtheoretical plates. Separate equilibrations of the samplebetween the stationary and mobile phase occur in these"plates". The analyte moves down the column by transferof equilibrated mobile phase from one plate to the next.BadResolutionGoodResolution理論板數 (number of balance)Greater theoretical plates Better separation resolution 29
Assessment of Column Efficiencytwice the distance between the two peaksResolutionindex(R) sInjectionsum of the base width of the two peaks2(t B - t A ) WA W BtRtBPeak width athalf peak heighttAW1/2APeakbroadeningBWWAWBElution Time30
Theoretical Plate Number—ResolutionA measure of separation efficiency: How many times theAnalyte mobile Analytestationary equilibrium is achievedN tR2σ2 tR σ tR N 16 W 22tR -σtR σW1/2 2.355σPeak broadening may beexpressed by variance 2.355 t RN peak width at half peak height retention distance width at half peak height tR2W 4σ2 5.54 W1/2tRW2Retention time: measure ofeffective column volume foranalyteBase width31
Height Equivalent to a Theoretical Plate(HETP) Length of a column necessaryfor the attainment of compounddistribution equilibrium (measurethe efficiency of the column).HETP length of the columnNh: Reduced plate heighth HETPpartical diameter(µm)Illustrate the effect of varying theflow rate of the mobile phase onthe efficiency of separationprocessInsufficient time forequilibriumHETPSeriousdiffusionOptimal flow rate for a specificcolumn and a mobile phaseFlow Rate (ml/min)32
Qualitative Analysis By comparison with known components, retention time(Distance) is used for identification of a component of a mixture.4.4 mintest retention timeRelative retention reference retention time4.4Relative retention 1.33.43.4 min33
Quantitative Analysis50.5 mm20.0 mmStandard0.1 ml of the internal standard (barbitone, 5.0 mmol/l)was added to 1.0 ml of sample.20 uL of the mixture was injectedWhat is the concentration of phenobarbitone?34
Injection 2Injection 1What is the concentration oftest sample?Reference: PropanolStandard: Ethanol35
Reference:EthanolConc.Peak anol/Propanol2.001.50數列1線性 (數列1)1.000.500.000510152025Ethanol Conc.36
Partition ChromatographyBMB 11.5Partition chromatography is based on differences incapacity factors and distribution coefficients of theanalytes using liquid stationary and mobile phases. Normal/Reverse Phase Chromatography Ion-Exchange Chromatography Gel Filtration Chromatography Affinity Chromatography37
Normal-Phase HPLCAdsorption of analytes on the polar, weakly acidic surfaceof silica gelOOOOOOOOOS iS iOOOOOS iS iS iOOOOS iS iOOOOS iS iS iS iS iOO HO HO HO HO HOOOO Stationary Phase.: Silica (pH 2-8), Alumina (pH 2 - 12),Bonded Diol, and NH2 Mobile Phase: Non-polar solvents (Hexane, CHCl3) Applications: Non-polar and semi-polar samples; hexanesoluble; positional isomers.38
Normal Phase Liquid ChromatographyPolar solutes elute later than non-polar lypophilic ones.39
Reversed-Phase HPLCPartition of analytes between mobile phase and stagnantphase inside the pore space adsorption on the surface ofbonded phaseC8C18 Stationary Phase: Hydrophobic surfaces of moietiesbonded on silica (C18, C8, C5, Phenyl, CN) Mobile phase: Methanol or Acetonitrile and Water. Applications: 80% of all separations done on RP HPLC.40
“Reverse” Phase Liquid ChromatographyIn Reversed Phase separations organic molecules are separated basedon their degree of hydrophobicity. There is a correlation between thedegree of lipophylicity and retention in the column.41
Ion Exchange Liquid ChromatographyElution order in ion exchange chromatography is determined by the chargedensity (charge/radius) of the hydrated ion. In organic acids and bases theelution order is determined by their pKa or pKb (strength of acid or base).42
Different Types of Ion Exchange ResinsCation exchangerCharge of Analyte charge- chargeAnion exchanger.43
Gel Permeation Chromatography -Molecular Sieve ChromatographyThe separation is based on the moleculesize and shape by the molecular sieveproperties of a variety of porous material44
Gel Permeation Chromatography (GPC) Also known as ‘size exclusion chromatography’and ‘gel filtration chromatography’ Separates molecules on the basis of molecularsize Separation is based on the use of a porousmatrix. Small molecules penetrate into the matrixmore, and their path length of elution is longer. Large molecules appear first, smaller moleculeslater45
Mass measurement by Gel PermeationChromatographyAB 3.4Vo: Void volume(outer volume)Vt: Bed volume(total volume)Vi: Gel inner volume)Ve: Effluent volume (Elution volume of the desired protein)Ve Vo KdxViVi Vt-VoKd Ve-VoVt--VoKd: partition constant of solutebetween gel matrix and solvent46
Medium massLargemassSmallmassElutiontime47
Determination of MassElution volumeThe elution volume is approximately alinear function of the logarithm of therelative molecular massLog (Relative Molecular Mass)48
Affinity Chromatography Affinity chromatography is based on a (not necessarily biologically relevant) interactionbetween a protein of interest, and a ligandimmobilized on a stationary phase substrate orproduct analogue– Antigen by Antibody:– Enzyme by Inhibitor /Substrate /Cofactor/coenzymeSpecific protein
polar? Example: Thin-Layer Chromatography. 19 Liquid-Liquid Chromatography Partition chromatography Adsorption chromatography Gel filtration chromatography Affinity chromatography Ion-exchange chromatography Example: High Performance Liquid Chromatography Partition of a solute between two immiscible liquid phases . 20 High Performance Liquid Chromatography