Transcription
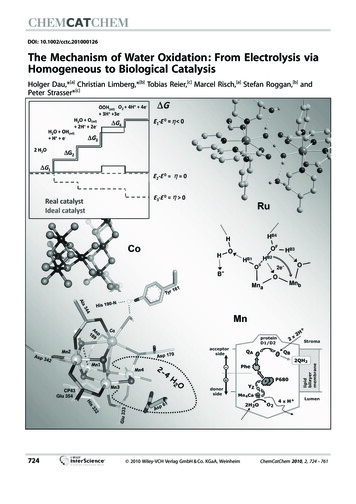
DOI: 10.1002/cctc.201000126The Mechanism of Water Oxidation: From Electrolysis viaHomogeneous to Biological CatalysisHolger Dau,*[a] Christian Limberg,*[b] Tobias Reier,[c] Marcel Risch,[a] Stefan Roggan,[b] andPeter Strasser*[c]724 2010 Wiley-VCH Verlag GmbH & Co. KGaA, WeinheimChemCatChem 2010, 2, 724 – 761
Striving for new solar fuels, the water oxidation reaction currently is considered to be a bottleneck, hampering progress inthe development of applicable technologies for the conversionof light into storable fuels. This review compares and unifiesviewpoints on water oxidation from various fields of catalysisresearch. The first part deals with the thermodynamic efficiency and mechanisms of electrochemical water splitting by metaloxides on electrode surfaces, explaining the recent concept ofthe potential-determining step. Subsequently, novel cobaltoxide-based catalysts for heterogeneous (electro)catalysis arediscussed. These may share structural and functional propertieswith surface oxides, multinuclear molecular catalysts and thecatalytic manganese–calcium complex of photosynthetic wateroxidation. Recent developments in homogeneous water-oxidation catalysis are outlined with a focus on the discovery ofmononuclear ruthenium (and non-ruthenium) complexes thatefficiently mediate O2 evolution from water. Water oxidation inphotosynthesis is the subject of a concise presentation ofstructure and function of the natural paragon—the manganese–calcium complex in photosystem II—for which ideas concerning redox-potential leveling, proton removal, and O Obond formation mechanisms are discussed. The last part highlights common themes and unifying concepts.Introductiontensified, as it can serve as an important inspiration andbenchmark for the development of artificial water-oxidationcatalysts.Herein we will discuss and compare concepts and recentfinding on the mechanism of water oxidation obtained in thefollowing separate research communities:* Heterogeneous electrocatalysis at electrode surfaces (section 1),* heterogeneous catalysis by oxidic bulk materials in colloidal form or deposited on electrodes (section 2),* homogeneous catalysis by transition metal complexes(section 3),* biocatalytic water oxidation in photosynthesis (section 4).Rather than approaching the impossible, that is, a fully comprehensive review, we focus on recent progress and strive toaddress common themes and potentially unifying concepts.In recent years, the increased interest in direct conversion ofsolar energy into storable fuels has led to intensified researchefforts with respect to artificial photosynthesis.[1] A successfulimitation of the natural energy-storing process requires thecombination of several distinct processes, including light harvesting, charge separation, electron transfer, and water oxidation (O2 evolution), as well as the fuel-forming reaction. Whereas a close adaption to the biological process of photosynthesiswould involve the transformation of carbon dioxide into achemical compound of higher energy content, at present thegeneration of molecular hydrogen (H2) by means of proton reduction seems to be a more realistic proposition for storingsolar energy by artificial photosynthesis.The electrons and protons required for fuel generationshould be obtained from oxidation of water to oxygen, thereby converting the whole process into an overall water-splittingscheme, where hydrogen is the targeted fuel. Hydrogen itselfcan be utilized as a fuel. Moreover, solar hydrogen will be thekey for the realization of a number of related renewable fuelproduction processes, such as hydrodeoxygenation of lignocellulosic biomass into fuels and chemicals, or the reduction ofCO2 into methanol or other carbonaceous fuels. However, notonly the generation of molecular hydrogen from water requires water oxidation. For all substances that today are typically considered as fuels, it holds that utilization of the chemically stored energy involves the reduction of molecularoxygen, that is, the transfer of electrons from the fuel tooxygen. For carbonaceous fuels, this process is coupled to CO2formation and, in most cases, also to H2O formation. In fuelgeneration, a source for these electrons is needed, and wateris the one and only truly attractive candidate; every schemefor sustainable, large-scale production of non-fossil fuels willeventually involve utilization of water as a raw material.Water oxidation is currently considered a major bottleneck,hampering progress in the development of applicable devicesfor the conversion of light into storable fuels. Intense researchefforts are being pursued to develop both heterogeneous andmolecular catalysts that enable water oxidation at potentialsclose to the thermodynamic limit. Research into the biologicalprocess of photosynthetic water oxidation has also recently inChemCatChem 2010, 2, 724 – 7611. Electrochemical Water SplittingElectrolysis, the electrocatalytic splitting of liquid water into hydrogen and oxygen using electricity, is the longest-studied catalytic way to oxidize water, predating even the developmentof the concept of chemical catalysis. The overall reaction, catalyzed at the electrified solid–liquid–gas three-phase interfaceof the anode and the cathode, is given byH2 OðlÞ ! H2ðgÞ þ1 2 O2ðgÞð1Þ[a] Prof. Dr. H. Dau, M. RischFreie Universit t Berlin, Physics Dept., Molecular BiophysicsArnimallee 14, 14195 Berlin (Germany)Fax: ( 49) 30-838-56299E-mail: holger.dau@fu-berlin.de[b] Prof. Dr. C. Limberg, Dr. S. RogganHumboldt Universit t Berlin, Department of ChemistryBrook-Taylor-Strasse 2, 12489 Berlin (Germany)Fax: ( 49) 30-2093-6966E-mail: christian.limberg@chemie.hu-berlin.de[c] T. Reier, Prof. Dr. P. StrasserTechnische Universit t Berlin, Chemistry Dept.The Electrochemical Energy, Catalysis and Materials Science LaboratoryStrasse des 17.Juni 124, 10623 Berlin (Germany)Fax: ( 49) 30-314-22261E-mail: pstrasser@tu-berlin.de 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheimwww.chemcatchem.org725
H. Dau, C. Limberg, P Strasser et al.Holger Dau received his physics diploma in 1985 and doctoral degree in1989 in Kiel, Germany, working withU.-P. Hansen and at the Weizmann Institut (Rehovot, Israel, winter 1987/88).After postdoctoral work with K. Sauerat the UC Berkeley, USA, and H. Sengerin Marburg, Germany, he received hishabilitation degree at the Biology Department of Philipps University Marburg in 1994. Besides photosynthesisresearch in Marburg, he developed biotest applications at bbe Moldaenke GmbH (1997–1999). Since2000, he has been a full Professor at the Physics Department ofthe Free University in Berlin, Germany, where he investigates biological and synthetic metal sites with X-ray spectroscopy and complementary methods. His current focus lies on light-driven wateroxidation and H2 formation in biological and biomimetic systems.Christian Limberg was born in 1965 inEssen, Germany, and studied chemistryfrom 1985 until 1990 in Bochum, Germany. He earned his doctorate with A.Haas in 1992 and concluded postdoctoral work together with A. J. Downsat Oxford University, UK, with a furtherPhD thesis (D. Phil.). Having performedhis habilitation in Heidelberg (1995–1999) he moved to the TU Munich inGermany to lead the inorganic chemistry chair on behalf of W. A. Herrmann.In 2002, he accepted an offer to become full professor at the Humboldt-Universit t zu Berlin, Germany. His research is mainly concerned with oxo metal complexes, oxidation reactions and theirmechanisms (surfaces of metal–oxo catalysts as inspiration for molecular models; biomimetic O2 activation and oxidation) as well aswith the activation of small substrates utilizing dinuclear complexes.Tobias Reier received his Master ofScience (Dipl.-Ing.) in 2010 at the Technical University Berlin, Germany, inChemical Engineering. He is currentlyworking on his doctoral thesis at theTechnical University of Berlin in thegroup of Prof. Strasser. His research isfocused on a fundamental understanding of mechanistic aspects and structural effects of electrochemical watersplitting as well as the design of improved water-splitting electrocatalysts.726www.chemcatchem.orgMarcel Risch received his Bachelor ofScience (physics) at the Technical University of Darmstadt, Germany, in 2006and a Master of Science (physics) atthe University of Saskatchewan,Canada, in 2008. In 2009, he joined theBerlin International Graduate School ofNatural Sciences and Engineering (BIGNSE) and he is currently studying for aPhD in the group of Prof. Dau at theFree University of Berlin. The topic ofhis thesis is the characterization of anelectrochemical cobalt catalyst.Stefan Roggan graduated in chemistryin 2003 at the Ruprecht-Karls University of Heidelberg in Germany. He thenmoved together with his supervisorProf. Christian Limberg to the Humboldt-Universit t zu Berlin, where heperformed his doctoral studies. In2007, he received his PhD in chemistryand then joined Prof. T. Don Tilley’sgroup at the UC Berkeley, USA, as apostdoctoral fellow. After his returnfrom the US in 2009, he continued research in Christian Limberg’s group before starting to work as ascientist for Bayer Technology Services GmbH in Leverkusen, Germany.Peter Strasser is a full Professor at theChemical Engineering Division of theDepartment of Chemistry at the Technical University of Berlin. His researchinterests focus on materials and catalysis for electrochemical (energy) technologies. Prior to his appointment, hewas an Assistant Professor at the Department of Chemical and Biomolecular Engineering at the University ofHouston, USA. From 2001 to 2004, heserved as senior member of staff atSymyx Technologies, Inc., Santa Clara, CA, developing and utilizingcombinatorial and high throughput screening methodologies inheterogeneous and electrocatalysis. In 1999, Peter Strasser obtained his doctoral degree in Physical Chemistry and Electrochemistry from the Fritz-Haber-Institute of the Max-Planck-Society,Berlin, under the direction of the 2007 Chemistry Noble Laureate,Professor Gerhard Ertl. He studied chemistry at Stanford University,USA, the University of T bingen, Germany, and the University ofPisa, Italy, and received his diploma degree (MS) in chemistry in1995. 2010 Wiley-VCH Verlag GmbH & Co. KGaA, WeinheimChemCatChem 2010, 2, 724 – 761
The Mechanism of Water OxidationIn the future, water electrolysis could play a key role in sustainable energy conversion and storage infrastructure.[2] Firstobserved in 1789 in a transient discharge experiment by vanTroostwijk,[3] then possibly observed by Volta using one of hispiles[4] and finally made known to a wider audience in 1800 byNicolson and Carlisle,[5] it largely remained a scientific curiositythroughout the 19th century. During the early decades of the20th century, water electrolysis was industrialized and served toproduce very pure hydrogen for industrial uses, such as ammonia production or early petroleum refining.[6] Later in the20th century, materials and system improvements resulted insignificant increases in net efficiencies of electrolyzers above50 %. Alkaline water electrolysis, where hydroxide anions arethe major charge carrier in the liquid ion-conducting electrolyte, became a technology of choice for production of oxygenand hydrogen for life-support systems in anaerobic environments, such as submarines. The use of separator materials (diaphragms) aided in the design of more-compact electrolyzercells with improved separation of hydrogen and oxygen gas.[7]Later on, high-pressure alkaline electrolyzers were designedand commercialized. Work by Beer resulted in the technologically important dimensionally stable anode (DSA),[8] which remains the basis of electrolyzer anodes to date. In the DSA, catalysts are supported directly on metallic titanium as a thin film.Space exploration drove the development of polymer electrolyte membrane (PEM) electrolyzers for acidic media thatshowed very similar system architectures as hydrogen PEMfuel cells (PEMFCs).[9] Membrane materials such as perfluorosulfonic acid (Nafion ) allow the conduction of protons across athin polymer electrolyte layer. Advantages of proton-conducting PEM devices over liquid alkaline systems include highersafety and reliability, because no caustic soda is circulated inthe cell, greater energy efficiency, sustainability to high differential gas pressures, higher hydrogen production rates, and amore-compact cell design.[9a] Drawbacks relate to the use ofmore expensive noble metal anode catalyst materials, such asRu or Ir. Current developments in the field of electrolysis include the coupling of water electrolysis with photovoltaic solarenergy conversion[10] into an integrated direct photoelectrocatalytic (PEC) water-splitting technology.[11] PEC devices promisemore-compact electrode and possibly device architectures, yetrequire multifunctional hybrid electrode materials. Low solarconversion efficiencies combined with electrocatalytic overpotential losses largely limit the efficiencies of today’s PEC devices to below 5 %. Another current development centers on thesynthesis of practical alkaline polymer electrolyte membranes.This technology would combine the advantages of the PEMtechnology with the benefits of a mature alkaline electrolyzerindustry, in particular with regards to the use of less expensivenon-noble metal electrode materials. Finally, the integration ofelectrolyzer and fuel-cell functionalities into one single unitizedregenerative fuel-cell device is the subject of current researchand development efforts.[12] A regenerative fuel cell/electrolyzersystem offers the prospect of a two-way conversion of chemical and electrical energy using bifunctional electrodes with theadvantage of further increased system simplicity and compactness.ChemCatChem 2010, 2, 724 – 761Today, water electrolysis is frequently cited as a clean cornerstone production technology in a largely hydrogen-based sustainable energy infrastructure (hydrogen economy[13]). Electrolysis could serve to liberate hydrogen from a range of chemicals, not only from water. Also, being a scalable technology,electrolysis would allow the initial deployment of smaller-scaleelectrolysis units at comparable efficiencies to large-scale electrolyzers.[13b–d] The vision of a broader hydrogen-based energyeconomy, however, still faces a large number of serious technical and nontechnical road blocks.[13d] Among the scientific andtechnical challenges, the need for advanced materials is criticaland will likely require a large amount of additional basicenergy materials and catalysis research.Hydrogen is an important industrial feedstock for petroleumrefining and fertilizer industry. However, electrolysis accountsonly for about 4 % of global hydrogen production[9b, 13b–d, 14] .The recent surge of interest and investments in renewableelectricity through solar–thermal, solar–electric (photovoltaic),or wind farms, combined with the emerging interest in electromobility concepts for urban centers, has raised a substantialamount of awareness about pressurized electrolytic hydrogenfor storage of excess renewable electricity. As a results, hydrogen–wind hybrid power plants are currently being commercialized at a number of locations worldwide.[15] On a smaller scale,more and more consumer wind and photovoltaic units havebeen deployed in accordance with energy policies that offergenerous subsidies for renewable electricity fed back into thegrid. Once these subsidies are cut back and grid electricityprices continue to rise, a growing interest in home energy storage units, possibly based on water electrolysis and compressedhydrogen, will result.The overall electrochemical water splitting is divided intotwo half-cell redox reactions. The reduction process at thecathode (hydrogen evolution reaction, HER) proceeds according to2 Hþ þ2 e ! H2ð2Þwhile the oxidation process (oxygen evolution reaction, OER)at the anode of the electrolyzer is[16]H2 O ! 1 2 O2 þ2 Hþ þ2 e ð3ÞIn the total process, two electrons are transferred per formulaor a total charge of 192 928 C ( 2 F) crosses the electrocatalytic interface per mole of water converted.We will focus herein on the mechanistic aspects of watersplitting (electrolysis) itself. Photoelectrochemical water splitting, whereby a photon-absorbing component is directly coupled with a gas-evolution electrocatalyst, is beyond the scopeof this review. A recent article has highlighted photoelectrochemical water splitting in the context of photoelectrochemical solar fuels.[11] 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheimwww.chemcatchem.org727
H. Dau, C. Limberg, P Strasser et al.1.1. The thermodynamic efficiency of electrochemical watersplittingThe equilibrium thermodynamics of galvanic and electrolysiscells extends the set of thermodynamic variables of a chemicalprocess that does not involve free charges, an electric potential E, or the difference of two potentials, the voltage V.[17] Cellvoltages at constant pressure P and temperature T are relatedto thermal energy quantities by the amount of electricity transferred nF, where n denotes the number of electrons transferredand F is the Faraday constant.Water splitting (ws) according to Equation (1) at standardtemperature and pressure (STP) is associated with a molar enthalpy change of reaction, herein denoted as DHows,298, which isequal to DHof,H2 OðlÞ ,298, the heat of formation of one mole ofliquid water at temperature 298 K. The absolute value of thesequantities is 286 kJ mol 1 (water) or 2.96 eV. The quantityDHows,298 is also referred to as the higher heating value DHoHHV,298of one mole hydrogen. The change in Gibbs free energy of reaction (1) DGows,298 is 237 kJ mol 1 (water) or 2.46 eV. Note: Different energy units are used in the reviewed fields and we donot use consistently the same units but rather those most convenient in the specific context. For a rough transformation ofenergy units: 1 eV 100 kJ mol 1 25 kcal mol 1.At equilibrium, the minimum electrical energy, 2 F Vrev, required to split one mole of water under STP is related to theGibbs energy asDGows,298 ¼ 2 F V orev,298ð4ÞConsidering only absolute values of cell voltages, V orev,298 1.23 V at STP and is referred to as the reversible electrolysis cellvoltage at STP.The total energy required to split one mole of water at STPis given byDHows,298¼ DHoHHV,298¼ DHof,H2 OðlÞ ,298¼ DGows,298þ T DSows,298ð6ÞoHHV,298orev,298where V 1.48 V. The voltage difference between Vand V oHHV,298 implies that water electrolysis below 1.48 V andabove 1.23 V is an endothermic process at STP. Hence, neglecting ohmic and other loss processes, an electrolysis cell operated below 1.48 V would cool down during electrolysis or wouldrequire a heat supply to operate isothermally.At elevated temperatures, V orev,T drops based on Equations (4)and (5), whereas DHoHHV,T and V orev,T increase according to[18–19]DHoHHV,T ¼ ¼ DHof,H2 OðlÞ ,T þ ðHoH2 OðlÞ ,T of,H2 OðlÞ ,298Þ ¼ 2 F V oHHV,T728www.chemcatchem.orgDHTN;T;p ¼DHHHV;T;ðp pw Þ þ 1:5 pw HH2 OðlÞ ;T;pw HHo 2 OðlÞ ;298ð p pw Þð8Þ¼ 2 F VTN;T;pThe first term of the central expression is the higher heatingvalue of the electrolysis process at temperature T, and gaspressure (p pw), while the second term accounts for the stoichiometric evaporation of water from STP to operating conditions.Owing to a variety of energy and efficiency losses, cell voltages in practical electrolysis cells[9b] are generally above boththe thermoneutral voltage VTN[18] and the higher heating valuevoltage VHHV. That is, excess thermal energy generally leads toheating of the electrolysis cell.The efficiency of an isothermal electrolysis cell operated at acell voltage V oop,T relates its chemical energy output to its electrical energy input, 2 F Voop,T. The energy output may be considered in two separate ways. Firstly, the maximum reversiblework (Gibbs free energy) DG from reconverting hydrogen andoxygen, for instance in a fuel cell, may be of interest andhence a faradaic (voltage) efficiency[9b] of electrolysis, ho298;Faradaic ,can be written asho298;Faradaic ¼oDGows;298 Vrev;298¼ o2 F VopVop;298ð9Þð5Þwhere the last term at STP is 49 kJ mol 1 and corresponds tothe heat required from the surroundings. Thus, a higher-heating-value voltage, VHHV,[18] or an equivalent amount of electricity, 2 F VHHV, can be related to the water splitting energy according toDHoHHV,298 ¼ 2 F V oHHV,298where HoH2 OðlÞ ,T is the molar enthalpy of water at standard pressure and temperature T. As a result, the voltage range of endothermic water splitting broadens.[18]The formalism above assumed the electrolytic production ofperfectly dry hydrogen and oxygen gas. Taking into accountthe presence of a water-vapor partial pressure pw at a totalpressure p of hydrogen and oxygen, a total energy for splittingof one mole of water into humidified gases can be derived,which is associated with a thermo-neutral voltage VTN according to[18–19]ð7ÞThis quantity essentially represents the reciprocal value ofthe voltage efficiency of a fuel cell[20] with Vop replaced by theexperimentally observed output cell voltage of a fuel cell. Thefaradaic efficiency can never be larger than one. Secondly, hydrogen and oxygen may be viewed in terms of their heatingvalue and hence a thermal efficiency of an electrolysis cell,ho298;thermal , is written as[18]ho298;thermal ¼ooDHHHV;298VHHV;298¼ o2 F VopVop;298ð10ÞThis efficiency has become widely accepted even though itwould never be possible to recover the entire higher heatingvalue of hydrogen in an energy application. In principle,ho298;thermal can be larger than unity.[6, 18–19] It is the reciprocalvalue of the reversible thermodynamic efficiency of a fuelcell.[20] Typical values of hoT;thermal of industrial electrolyzers rangebetween 60 % to 80 % at a given current density (typically1 A cm 2) and at 363 K (90 8C) and 0.1 MPa. 2010 Wiley-VCH Verlag GmbH & Co. KGaA, WeinheimChemCatChem 2010, 2, 724 – 761
The Mechanism of Water OxidationEquations (9) and (10) provide a measure of the efficiency ofoverall water splitting. They are generally valid, regardless ofwhether it is an electrocatalytic process at an inorganic electrode, a biocatalytic process, or a catalytic pathway involvinghomogeneous organometallic catalysts. In a ‘translation’ of theelectrochemical terminology, anode and cathode potentialscorrespond to the midpoint redox potentials of the oxidant(anode potential) and reductant (cathode potential) that areused to drive the OER or HER. Similarly, in electrochemical, homogeneous, and biological catalysis, research typically considers only half-cell reactions, as is also the case in the mechanistic discussions of water oxidation (section 1.2). For half-cell reactions, however, Equations (9) and (10) are not directly applicable.Equation (9) may be reformulated asho298;Faradaic ¼orev;298VoVrev;298þ OPanode þ OPcathodeð11Þwhere OPanode and OPcathode are the absolute values of the overpotentials of the OER (anodic reaction) and HER (cathodic reaction), respectively. These overpotentials can be calculated inhomogeneous and biocatalysis as the difference between themidpoint potential of the oxidant (or reductant) and the OER(or HER) equilibrium potential. To quantify the efficiency of thehalf-cell reaction, we now assume for OER (or HER) thatOPcathode (or OPanode) equals zero. The thereby-obtained value ofhoOER (hoHER ) represents a meaningful measure of the energeticefficiency of the catalysis of water oxidation (hydrogen evolution) in electrochemical, homogeneous, and biocatalysis. For acombination of two half-cell reactions, calculation of the overall efficiency ho from the two half-cell efficiencies is straightforward. However, ho is not equal to the product of hoOER and hoHERbut to [(hoOER ) 1 (hoHER ) 1 1] 1.1.2. Reaction mechanisms of electrochemical water splittingAs described above, the electrochemical splitting of water involves two concurrent catalytic half-cell reactions accordingEquation (2) (HER) and Equation (3) (OER). Also shown aremechanistic schemes for either reaction process, which havebeen proposed in literature in the past. Clearly, oxygen evolution being a 4 electron process is more complex as comparedto the evolution of hydrogen and involves several surface adsorbed intermediates. As either process may contribute to voltage and efficiency losses, a discussion of the electrocatalysis ofwater splitting should in principle touch upon both the HERand OER. Work on biocatalytic water splitting has focusedeither on photosystem II (oxygen evolution), as discussed insection 4, or hydrogenase enzymes (hydrogen evolution),which are not discussed herein. Similarly, research concerninghomogeneous catalysis of water splitting has involved systemsliberating either hydrogen or oxygen (or, in rare cases, both;see section 3.2 for comparison). In electrochemical environments, HER on selected noble metals shows extremely high exchange current densities, j0, which is a measure of the intrinsicChemCatChem 2010, 2, 724 – 761turnover frequency of an electrochemical half-cell process. OnPt electrodes (Figure 1), for instance, HER shows values of j0 ofup to several hundred A cm 2,[21] resulting in a very reversiblehalf-cell electrode with high forward and backward reactionFigure 1. Catalytic reactions associated with electrochemical water electrolysis. An external voltage, symbolized by the battery symbol, drives the evolution of hydrogen at the cathode (HER) and the evolution of oxygen at theanode (OER). Protons are migrating from anode to cathode. Both mechanisms are depicted assuming a sequence of proton-coupled single-electronredox reactions. While HER shows one adsorbed intermediate, OER hasthree. The spheres symbolize a metal electrode, for example, Pt, coveredwith an oxide layer. The numbers in parentheses refer to chemical equationsgiven in the text.rates. However, OER on Pt, the catalytically most active element for this reaction, exhibits values of j0 on the order of10 9 A cm 2, which is why much recent work has shiftedtoward OER kinetics. Therefore, the OER is our focus here andalso in upcoming sections.In electrocatalysis, a fundamental concept in characterizingcatalytic processes on surfaces is the overpotential.[22] Overpotential is the deviation of the observed output cell voltage Vop(galvanic cell) or the applied cell voltage Vop (electrolysis) usedin Equations (9) and (10) from the thermodynamic reversiblecell voltage Vrev [Equation (4)]. The overpotential is generallyconsidered a kinetic phenomenon and splits into various contributions such as the ohmic voltage loss (ohmic overpotential)or mass-transport limitations (transport overpotential) duringthe flow of charge.[22] In the context of catalysis, “reaction overpotentials” relate to a kinetic hindrance of individual elementary reaction steps due to activation barriers. On that basis, reaction overpotential is a function of the flowing current and isthought to be linked to the slowest of the elementary reactionsteps, the rate-determining step (RDS), with the highest kineticactivation barrier.Despite much work on the OER, mechanistic models and hypotheses of the sequence of elementary steps based onatomic-scale experiments have remained scarce. Over thecourse of five decades, proposed mechanisms, conclusions asto the RDS, and experimental approaches have changed onlylittle. A severe hurdle to more direct atomic-scale insight intoOER is the fact that reactive intermediates have largely eludeddirect spectroscopic observation. Most early mechanistic workrelies on classical current–potential–time analyses and the derived Tafel slopes. Tafel slopes are derived from semi-logarithmic potential current plots and have been frequently used toobtain information on the mechanism and the rate-determin- 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheimwww.chemcatchem.org729
H. Dau, C. Limberg, P Strasser et al.ing step of the overall half-cell reaction. An early mechanisticscheme of electrochemical water splitting based on experiments on RuO2-based electrodes in acidic environments is outlined by Equations (12)—(15):[23]SþH2 O ! S OHþHþ þe ð12ÞS OH ! S OþHþ þe ð13ÞS OHþS OH ! S OþSþH2 Oð14ÞS OþS O ! O2 þ2 Sð15ÞIn this mechanistic hypothesis, S denotes a catalyticallyactive surface site. Equations (12), (13), and (15), on the onehand, and (12), (14), and (15), on the other, represent parallelreaction pathways. This mechanism and very similar schemeswere proposed for both well-defined Ru(110) single-crystaloxide surfaces[23d, 24] and compact RuO2 films.[23d] RuO2 singlecrystals were reported with a near 60 mV decade 1 and a120 mV decade 1 Tafel slope at low and high overpotentials, respectively.[23d, 24] On that basis, reaction (12) was thought to bethe RDS of the sequence (12)–(13)–(15) at high electrode potentials. An additional second chemical step, such as the hypothetical rearrangement of the S OH species according toEquation (16):[24]S OH ! S OH*de 1.[23d, 27c, d] Similar to the single crystal work, reaction (16) wasproposed for these films as a second elementary step. Lowtemperature Ru oxide films showed high BET surface areavalues combined with higher values of q*. The OER activitywas high for these films and the Tafel slope ranged in the40 mV decade 1 range. Hence, an electrochemical reaction (13)was now proposed as the rate-determining step and the fullmechanistic sequence thus becomes (12)–(13)–(15).Binary and ternary mixed oxides containing Ir, Sn, and Rushowed their own distinct complex Tafel slope patterns withno clear trend or controlling parameter.[25, 27c, d, g, 29] A number ofrecent studies focused on the effect of particle size on the OERmechanism for RuO2 and Ru1 xCoxO2 mixed oxides.[30] The authors used differentially pumped mass spectrometry (DEMS) todirectly correlate the evolution of molecular oxygen and its faradaic current profile. They
structure and function of the natural paragon—the manga-nese-calcium complex in photosystem II—for which ideas con-cerning redox-potential leveling, proton removal, and O O bond formation mechanisms are discussed. The last part high-lights common themes and unifying concepts. [a] Prof. Dr. H. Dau, M. Risch