Transcription
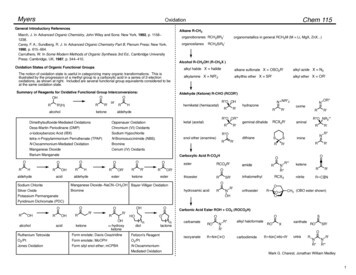
MyersChem 115OxidationGeneral Introductory ReferencesAlkane R-CH3March, J. In Advanced Organic Chemistry, John Wiley and Sons: New York, 1992, p. 1158!1238.Carey, F. A.; Sundberg, R. J. In Advanced Organic Chemistry Part B, Plenum Press: New York,1990, p. 615!664.Carruthers, W. In Some Modern Methods of Organic Synthesis 3rd Ed., Cambridge UniversityPress: Cambridge, UK, 1987, p. 344!410.organoboranes RCH2BR2'organosilanesThe notion of oxidation state is useful in categorizing many organic transformations. This isillustrated by the progression of a methyl group to a carboxylic acid in a series of 2-electronoxidations, as shown at right. Included are several functional group equivalents considered to beat the same oxidation state.Summary of Reagents for Oxidative Functional Group Interconversions:OOOHRR'(H)alcoholR'orketonealkyl halide X halidealkane sulfonate X OSO2R'alkyl azide X N3alkylamine X NR'2alkylthio ether X SR'alkyl ether X OR'Aldehyde (Ketone) R-CHO (RCOR')HRRCH2SiR3'Alcohol R-CH2OH (R-CH2X )Oxidation States of Organic Functional GroupsRorganometallics in general RCH2M (M Li, MgX, ZnX.)hemiketal (hemiacetal)N NR''2R''O OHRaldehydehydrazoneR'RR''O OR'''Dimethylsulfoxide-Mediated OxidationsOppenauer OxidationDess-Martin Periodinane (DMP)Chromium (VI) Oxidantso-Iodoxybenzoic Acid (IBX)Sodium Hypochloritetetra-n-Propylammonium Perruthenate (TPAP)N-Bromosuccinimide (NBS)N-Oxoammonium-Mediated OxidationBromineManganese DioxideCerium (IV) Oxidantsketal erOR'RRaminalSSimineRR'R'R''O NR2'''RR''OOOR'RCX2R'R'Carboxylic Acid R-CO2HOOHR'enol ether (enamine)Barium ManganateORgeminal dihalideRketoneesteresterSodium ChloriteManganese Dioxide!NaCN!CH3OH Bayer-Villiger OxidationSilver HorthoesterPotassium PermanganateR'''R''R'keteneRRCX3Ohydroxamic acidNONR''OOR'R'ORCO2R'OR''NoximenitrileR'R C NORCH3 (OBO ester shown)OOPyridinium Dichromate ketoneOHOCarbonic Acid Ester ROH CO2 (ROCO2H)OdiolnRuthenium TetroxideForm enolate; Davis OxaziridineFetizon's ReagentO2/PtForm enolate; MoOPHO2/PtJones OxidationForm silyl enol ether; mCPBAN-Oxoammonium-carbamatelactoneMediated OxidationOOnisocyanateRONR'R''R N C Oalkyl haloformateROSXxanthateROSR'OcarbodiimideR N C N R'ureaRNR''R'NR'''Mark G. Charest, Jonathan William Medley1
MyersChem 115OxidationOOHR'RketoneRR'(H)alcohol Pummerer RearrangementOorHO CH3 OHH3CHHRaldehydeH3CDimethylsulfoxide-Mediated OxidationsOHHO CH3 OHH3CHCF3CO2Ac, Ac2O2,6-lutidineO ReviewsTidwell, T. T. Organic Reactions 1990, 39, 297!557.H3CGeneral Mechanism Dimethylsulfoxide (DMSO) can be activated by reaction with a variety of electrophilic reagents,including oxalyl chloride, dicyclohexylcarbodiimide, sulfur trioxide, acetic anhydride, and Nchlorosuccinimide. The mechanism can be considered generally as shown, where the initial step involveselectrophilic (E ) attack on the sulfoxide oxygen atom. Subsequent nucleophilic attack of an alcohol substrate on the activated sulfoxonium intermediateleads to alkoxysulfonium salt formation. This intermediate breaks down under basic conditions tofurnish the carbonyl compound and dimethyl sulfide. –(CH3)2S O(CH3)2S XE SOPhOOHHO CH3 OHH3CHOOAc 60%H3COH–AcOOS PhS Ph Schreiber, S. L.; Satake, K. J. Am. Chem. Soc. 1984, 106, 4186!4188.Swern Procedure Typically, 2 equivalents of DMSO are activated with oxalyl chloride in dichloromethane at orbelow –60 C. Subsequent addition of the alcohol substrate and triethylamine leads to carbonyl formation. The mild reaction conditions have been exploited to prepare many sensitive aldehydes.Careful optimization of the reaction temperature is often necessary. H HORHO CH3 OHH3CHTidwell, T. T. Synthesis 1990, 857!870.H–BH –RCO2–S PhOLee, T. V. In Comprehensive Organic Synthesis, Trost, B. M.; Fleming, I., Eds., PergamonPress: New York, 1991, Vol. 7, p. 291!303.OH3CBHuang, S. L.; Mancuso, A. J.; Swern, D. J. Org. Chem. 1978, 43, 2480!2482. RCH2OH B(CH3)2S XH H CH3 S RCH3O–CH2S OCH3H HR–H HR X–HOO3. (COCl)2, DMSO; Et3N(CH3)2S–78 " –50 COBnalkoxysulfonium ylideOH66% Methylthiomethyl (MTM) ether formation can occur as a side reaction, by nucleophilic attack ofan alcohol on methyl(methylene)sulfonium cations generated from the dissociation of sulfoniumylide intermediates present in the reaction mixture. This type of transformation is related to thePummerer Rearrangement.Evans, D. A.; Carter, P. H.; Carreira, E. M.; Prunet, J. A.; Charette, A. B.; Lautens, M. Angew.Chem., Int. Ed. Engl. 1998, 37, 2354!2359.NRO–H SCH3Fenselau, A. H.; Moffatt, J. G. J. Am. Chem. Soc. 1966, 88, 1762!1765.CH3NOOHNNClCH3N(COCl)2, DMSO;O ROH H2C S CH3TBSO2. 10% Pd/C, AcOH, EtOAcOOTBSO1. TBSCl, Im, DMAP, CH2Cl2HOEt3N, –78 CO99%100-g scaleOCHONClFang, F. G.; Bankston, D. D.; Huie, E. M.; Johnson, M. R.; Kang, K.-C.; LeHoullier, C. S.; Lewis, G.C.; Lovelace, T. C.; Lowery, M. W.; McDougald, D. L.; Meetholz, C. A.; Partridge, J. J.; Sharp, M. J.;Xie, S. Tetrahedron 1997, 53, 10953!10970.Mark G. Charest, Jonathan William Medley2
MyersCH3OCH3OCH3HOOR1CH3OChem 115OxidationCH3CH3OOHO(COCl)2, DMSO;NCH3HR1OCH3OCH3HCH3CH3OCH3EDC (CH3)2N (CH2)3 N C N CH2CH3 HClR1OORBzO94%OOOCH3FK506HOROTFA, pyrNCH3OTBDPSODMSO, EDCOHOBzOO80%OOCH3CH3OTBDPSOOOR1Et3N, –78 CHORCH3OCH3Hanessian, S.; Lavallee, P. Can. J. Chem. 1981, 59, 870!877.ORParikh-Doering ProcedureR TIPS, R1 TBS Sulfur trioxide-pyridine is used to activate DMSO.Jones, T. K.; Reamer, R. A.; Desmond, R.; Mills, S. G. J. Am. Chem. Soc. 1990, 112, 2998!3017. Ease of workup and at-or-near ambient reaction temperatures make the method attractive for largescale reactions.Pfitzner-Moffatt ProcedureParihk, J. R.; Doering, W. von E. J. Am. Chem. Soc. 1967, 89, 5505-5507. The first reported DMSO-based oxidation procedure. Examples Dicyclohexylcarbodiimide (DCC) functions as the electrophilic activating agent in conjunctionwith a Brønsted acid promoter.Ph Typically, oxidations are carried out with an excess of DCC at or near 23 C. Separation of the by-product dicyclohexylurea and MTM ether formation can limit usefulness.Ot-BuDMSO, DCCClTFA, pyrOH8 " 23 COBn2NH99.9% ee 95%99.9% ee Alternative carbodiimides that yield water-soluble by-products (e.g., 1-(3-dimethylaminopropyl)-3ethylcarbodiimide hydrochloride (EDC)) can simplify workup procedures.ClOHBn2NPhSO3 pyr, Et3N, DMSO190-kg scaleLiu, C.; Ng, J. S.; Behling, J. R.; Yen, C. H.; Campbell, A. L.; Fuzail, K. S.; Yonan, E. E.; Mehrotra, D.V. Org. Process Res. Dev. 1997, 1, 45!54.Ot-BuOH87%OSO3 pyr, Et3N,HHHOCorey, E. J.; Kim, C. U.; Misco, P. F. Org. Synth. Coll. Vol. VI 1988, 220!222.HOHBrHDMSO, CH2Cl2OHH0 " 23 COHCHOBrH99%HHDMSO, DCCOHCO2CH3 TFA, pyrOCH39 : 1 #, : %,#SH3CCH3HCHOCO2CH3OCH3CH3CHOH CO2CH3OCH3SH3CCH3H3CSemmelhack, M. F.; Yamashita, A.; Tomesch, J. C.; Hirotsu, K. J. Am. Chem. Soc. 1978, ans, P. A.; Murthy, V. S.; Roseman, J. D.; Rheingold, A. L. Angew. Chem., Int. Ed. Engl. 1999,38, 3175!3177.Mark G. Charest, Jonathan William Medley3
MyersChem 115OxidationDess-Martin Periodinane (DMP) Examples DMP has found wide utility in the preparation of sensitive, highly functionalized molecules. DMP oxidations are characterized by short reaction times, use of a single equivalent of oxidant,and can be moderated with regard to acidity by the incorporation of additives such as pyridine. DMP and its precurser o-iodoxybenzoic acid (IBX) are potentially heat and shock sensitive andshould be handled with appropriate care.Dess, D. B.; Martin, J. C. J. Am. Chem. Soc. 1983, 48, 4155!4156.H3CH3CH3C HTBSOHI KBrO365 C, 2.5 hCO2Hthen 23 C, 24 hO74% overall Ac2O AcOHDMPR1R2CHOH–AcOHAc OOIHDMPMyers, A. G.; Zhong, B.; Movassaghi, M.; Kung, D. W.; Lanman, B. A.; Kwon, S. TetrahedronLett. 2000, 41, 1359!1362. Use of other oxidants in the following example led to conjugation of the ",#-unsaturated ketone,which did not occur when DMP was used.OICH3HOAcslowIOAc R1R2C O AcOHOOOR1R2CHOH–AcOHR1R2Ac OOIHOIIOOCHR1R2fastIOCHR1R2OODess, D. B.; Martin, J. C. J. Am. Chem. Soc. 1991, 113, 7277!7287.SePolson, G.; Dittmer, D. C. J. Org. Chem. 1988, 53, 791!794.Meyer, S. D.; Schreiber, S. L. J. Org. Chem. 1994, 59, 7549!7552.R2O For the synthesis of sensitive -amino aldehydes from the corresponding alcohols, the use ofDMP suppresses epimerization.PhDMPPhOOHFmocHNwet CH2Cl2FmocHNH23 C99% ee99% ee 95%IBX Addition of one equivalent of water has been found to accelerate the alcohol oxidation reactionwith DMP, perhaps due to the formation of an intermediate analogous to II. It is proposed thatthe decomposition of II is more rapid than the initially formed intermediate I:R1DMP 100%Ac OAcOIOAcO85 CH(–)-7-deacetoxyalcyonin acetateHSeOOHOOverman, L. E.; Pennington, L. D. Org. Lett. 2000, 2, 2683!2686.OHIH3CHH3CHO AcOOIOHOOH3CHH3CTBSO89% overallPlumb, J. B.; Harper, D. J. Chem. Eng. News 1990, July 16, 3.2.0 M H2SO41. DIBAL2. DMPOCH3CH3PivOBoeckman, R. K.; Shao, P.; Mulins, J. J. Org. Synth. 1999, 77, 141!152.IH3CH3CCH3 R1R2C O AcOHH3CDEIPSOOOOTESO O1. DDQ, CH2Cl2, H2OCH3CH3 CH3CH3 H2. DMP, CH2Cl2, pyrHOTBSOTESO93% overallO Si(t-Bu)2OPMBOCH3OCH3OCH3CH3OTESTESOHOH3COHDEIPSOO OHCH3 CH3CH3 HH(–)-cytovaricinTBSOTESOHHOOTESCH3OO Si(t-Bu)2OCH3OCH3OCH3OTESTESOEvans, D. A.; Kaldor, S. W.; Jones, T. K.; Clardy, J.; Stout, T. J. J. Am. Chem. Soc. 1990, 112, 7001!7031.Mark G. Charest, Jonathan William Medley4
MyersChem 115Oxidation DMP oxidation in the presence of phosphorous ylides allows for the trapping of sensitivealdehydes. IBX is used as a mild reagent for the oxidation of 1,2-diols without C-C bond cleavage.H3C OHOH3COHDMP, CH2Cl2, DMSO PhCO2HCO2CH3AcOHOH3CIBX, DMSOCH3O2CPh3P CHCO2CH3H3C OAcO85%HOOHO94% (2.2 : 1 E,E : E,Z)Frigerio, M.; Santagostino, M. Tetrahedron Lett. 1994, 35, 8019!8022.Barrett, A. G. M.; Hamprecht, D.; Ohkubo, M. J. Org. Chem. 1997, 62, 9376!9378. Pyridines are not oxidized at a rate competitive with the oxidation of a primary alcohol.OHONHFmocDMPNHFmocHOHN 90%SCH3Myers, A. G.; Zhong, B.; Kung, D. W.; Movassaghi, M.; Lanman, B. A.; Kwon, S. Org. Lett. 2000, 2, DMP has been used to oxidize secondary acyclic and macrocyclic amides to the correspondingimides in moist DMSO/fluorobenzene at elevated temperature.MeNHHNOtBuO6.0 equiv DMPwet DMSO, PhF85 C, 3.5 hON99%SCH33337!3340.OCHOIBX, DMSOMeOHNNHFrigerio, M.; Santagostino, M. Tetrahedron Lett. 1994, 35, 8019!8022. IBX has been shown to form ",#-unsaturated carbonyl compounds from the correspondingsaturated alcohol or carbonyl compound. The reproducibility of the results of this and related IBX-mediated oxidations has been found tooften depend on the presence of water in the IBX employed (for a discussion, see: secret-ingredient.html)OtBu4.0 equiv IBXO86%OHNtoluene, DMSOONNicolaou, K. C.; Mathison, C. J. N. Angew. Chem., Int. Ed. 2005, 44, 5992!5997.H84%o-Iodoxybenzoic Acid (IBX)O The DMP precursor IBX is gaining use as a mild reagent for the oxidation of alcohols. A simpler preparation of IBX has been reported.ICO2Hoxone, H2OO70 C79-81%TIPSH2.0 equiv IBXTIPStoluene, DMSOHH87%OHOOHI6.0 equiv IBXOOOHtoluene, DMSOIBXOHFrigerio, M.; Santagostino, M.; Sputore, S. J. Org. Chem. 1999, 64, 4537!4538.O52%Nicolaou, K. C.; Zhong, Y.-L.; Baran, P. S. J. Am. Chem. Soc. 2000, 122, 7596!7597.Mark G. Charest, Jonathan William Medley5
MyersChem 115Oxidationtetra-n-Propylammonium Perruthenate (TPAP): Pr4N RuO4–CH3CH3 ReviewsH3CHOLey, S. V.; Norman, J.; Griffith, W. P.; Marsden, S. P. Synthesis 1994, 639!666.Griffith, W. P.; Ley, S. V. Aldrichimica Acta 1990, 23, 13!19.CH3OR'OOH3CCHOR 3CH3OOH3C59%H3C27-g scaleO However, perruthenate salts with large organic counterions prove to be mild and selectiveoxidants in a variety of organic solvents.CH3OR'OCH2Cl2, 23 CH3C Ruthenium tetroxide (RuO4, Ru(VIII)) and, to a lesser extent, the perruthenate ion (RuO4–,Ru(VII)) are powerful and rather nonselective oxidants.H3COTPAP, NMOCHOR 3CH3OOR cladinose, R' 3-N'-demethyl-3'-N-phenylsulfonyl desosamine In conjunction with a stoichiometric oxidant such as N-methylmorpholine-N-oxide (NMO), TPAPoxidations are catalytic in ruthenium, and operate at room temperature. The reagents arerelatively non-toxic and non-hazardous. To achieve high catalytic turnovers, the addition of powdered molecular sieves (to remove boththe water present in crystalline NMO and the water formed during the reaction) is essential.Jones, A. B. J. Org. Chem. 1992, 57, 4361!4367.H3C CH3H CH3OCH3OOTBS TPAP, NMO, CH Cl2 2 CH3OHHOO4Å MS, 23 CCH3OCH3OThe following oxidation state changes have been proposed to occur during the reaction:OOHTBSO Ru(VII) 2e– " Ru(V)78%H3C CH3H CH3OOTBSHHOOOHOOTBSO2Ru(V) " Ru(VI) Ru(IV)Julia-LythgoeOlefinationRu(VI) 2e– " Ru(IV)Griffith, W. P.; Ley, S. V.; Whitcombe, G. P.; White, A. D. J. Chem. Soc., Chem. Commun. 1987,1625!1627.OHOOOTPAP, CH2Cl2NTEOC23 CHBu4NTEOCN F–,HOCH3 H OTBS OOOCH3NCH3TESOH29%84%CH3OCH3OO CH3OCH3CH3CH3CH3( )-indolizomycinCH3O2CKim, G.; Chu-Moyer, M. Y.; Danishefsky, S. J.; Schulte, G. K. J. Am. Chem. Soc. 1993, 115, 30!39.HOCH3TPAP, NMO, CH2Cl24 Å MS, 23 CCH387%CH3TESOOCH3 H OTBS OOOOOHCH3CH3CH3OHH3CH3CCH3O4 Å MS, 23 COH3CH3C CH3H CH3OOTBSHHOOTPAP, NMO, CH2Cl2OOHTHF0 CH3C CH3H CH3OOTBSHHOOCH3OCH3O ExamplesOOH3C CH3H HOOAcHHOOOCH3OOH H OHOCH3HCH370%Ley, S. V.; Smith, S. C.; Woodward, P. R. Tetrahedron 1992, 48, 1145!1174.O CH3n-PrObryostatin 3OOOHOhmori, K.; Ogawa, Y.; Obitsu, T.; Ishikawa, Y.;Nishiyama, S.; Yamamura, S. Angew. Chem., Int. Ed.Engl. 2000, 39, 2290!2294.Mark G. Charest, Jonathan William Medley6
MyersChem 115OxidationN-Oxoammonium-Mediated Oxidation ExamplesTEMPO, NaOCl ReviewsOBnde Nooy, A. E. J.; Besemer, A. C.; van Bekkum, H. Synthesis 1996, 1153!1174.BocHNOHBobbitt, J. M.; Flores, C. L. Heterocycles 1988, 24, 509!533.Rozantsev, E. G.; Sholle, V. D. Synthesis 1971, 401!414.EtOAc : toluene : H2O(6 : 6 : 1)C6H11 N-Oxoammonium salts are mild and selective oxidants for the conversion of primary andsecondary alcohols to the corresponding carbonyl compounds. These oxidants are unstable andare invariably generated in situ in a catalytic cycle using a stable, stoichiometric oxidant.NaBr, NaHCO377%OBnBocHNOHC6H11 95% deLeanna, R. M.; Sowin, T. J.; Morton, H. E. Tetrahedron Lett. 1992, 33, 5029!5032.See also: Jurczak, J.; Gryko, D.; Kobrzycka, E.; Gryza, H.; Prokopoxicz, P. Tetrahedron 1998, 54,RX–R1 NOH OH R2O–HXR R3R2R3RN 1OH6051!6064.OHN-oxoammonium saltOOTBDPS–NORR1 NHOOH R2R1R1RR1OHR2NH3C CH3R1H3C CH3OOBHR2R1OOR1R H –H kuehneromycin ARN 1OHR NOR1PhSTEMPO, BAIB, CH2Cl2CH2OHGolubev, V. A.; Sen', V. D.; Kulyk, I. V.; Aleksandrov, A. L. Bull. Acad. Sci. USSR, Div. Chem.Sci. 1975, 2119!2126. 2,2,6,6-Tetramethyl-1-piperidinyloxyl (TEMPO) catalyzes the oxidation of alcohols to aldehydesand ketones in the presence of a variety of stoichiometric oxidants, including mchloroperoxybenzoic acid (m-CPBA), sodium hypochlorite (NaOCl), [bis(acetoxy)-iodo]benzene(BAIB), sodium bromite (NaBrO2), and Oxone (2KHSO5 KHSO4 K2SO4).H3CCH3NOCH3CHOHH3C CH3nitroxyl radicalH3COHH Selective oxidation of allylic alcohols in the presence of sulfur and selenium has beendemonstrated.disproportionationNOOJauch, J. Angew. Chem., Int. Ed. Engl. 2000, 39, 2764!2765. N-Oxoammonium salts may be formed in situ by the acid-promoted disproportionation of nitroxylradicals. Alternatively, oxidation of a nitroxyl radical or hydroxyl amine can generate thecorresponding N-oxoammonium salt.ROTBDPS98%Ganem, B. J. Org. Chem. 1975, 40, 1998!2000.Semmelhack, M. F.; Schmid, C. R.; Cortés, D. A. Tetrahedron Lett. 1986, 27, 1119!1122.Bobbitt, J. M.; Ma, Z. J. Org. Chem. 1991, 56, 6110!6114.2H23 C Three possible transition states have been proposed:RTEMPO, BAIB, CH2Cl2TEMPOPhS23 CCHO70%H3CCH2OHSePhTEMPO, BAIB, CH2Cl223 CH3CCHOSePh55%De Mico, A.; Margarita, R.; Parlanti, L.; Vescovi, A.; Piancatelli, G. J. Org. Chem. 1997, 62, 6974!6977.Mark G. Charest, Jonathan William Medley7
MyersChem 115OxidationTBSOManganese Dioxide: MnO2HTBSOHSAr ReviewsCahiez, G.; Alami, M. In Handbook of Reagents for Organic Synthesis: Oxidizing and ReducingReagents, Burke, S. D.; Danheiser, R. L., Eds., John Wiley and Sons: New York, 1999, p. 231!236.HO HOHOOAcHHHSArMnO2, acetone76%O HOHOOAcHFatiadi, A. J. Synthesis 1976, 65!104.Trost, B. M.; Caldwell, C. G.; Murayama, E.; Heissler, D. J. Org. Chem. 1983, 48, 3252!3265.Fatiadi, A. J. Synthesis 1976, 133!167. A heterogenous suspension of active manganese dioxide in a neutral medium can selectivelyoxidize allylic, benzylic and other activated alcohols to the corresponding aldehyde or ketone. The structure and reactivity of active manganese dioxide depends on the method of preparation.H3C CH3H CH3CH3CH3CH3 Active manganese oxides are nonstoichiometric materials (in general MnOx, 1.93 x 2)consisting of Mn (II) and Mn (III) oxides and hydroxides, as well as hydrated MnO2.HO Hydrogen-bond donor solvents and, to a lesser extent, polar solvents have been shown toexhibit a strong deactivating effect, perhaps due to competition with the substrate for the activeMnO2 surface.MnO2OHacetoneCH375%CH3OHH3C CH3H CH3CH3CH3O ExamplesCH3CH3H3C CH3MnO2H3C CH3CH3OHHOCH3OHOCH3CH3 95%1-kg scale Vinyl stannanes are tolerated.CH3CH3OEtMnO2H3C CH3CH3OEtCH2Cl2, 0 troneHaugan, J. A. Tetrahedron Lett. 1996, 37, 3887!3890.Salman, M.; Babu, S. J.; Kaul, V. K.; Ray, P. C.; Kumar, N. Org. Process Res. Dev. 2005, 9,302!305.H3C CH3CH3CH376%89%OAlvarez, R.; Iglesias, B.; Lopez, S.; de Lera, A. R. Tetrahedron Lett. 1998, 39, 5659!5662.van Amsterdam, L. J. P.; Lugtenburg, J. J. Chem. Soc., Chem. Commun. 1982, 946!947.EtO2CCO2EtOHCCHO1. DIBAL, C6H6CH374%HO CH3OHCH3H3COMnO2OH3CCH32. MnO2, CH2Cl2H3C Syn or anti vicinal diols are cleaved by MnO2.100%CH3CH3CH3Ohloff, G.; Giersch, W. Angew. Chem., Int. Ed. Engl. 1973, 12, 401!402.Cresp, T. M.; Sondheimer, F. J. Am. Chem. Soc. 1975, 97, 4412!4413.Mark G. Charest, Jonathan William Medley8
MyersChem 115OxidationOppenauer Oxidation ReviewBarium Manganate: BaMnO4 ReviewFatiadi, A. J. Synthesis 1987, 85!127.de Graauw, C. F.; Peters, J. A.; van Bekkum, H.; Huskens, J. Synthesis 1994, 1007!1017. Barium manganate and potassium manganate are deep green salts that can be used withoutprior activation for the oxidation of primary and secondary allylic and benzylic alcohols. A classic oxidation method achieved by heating the alcohol to be oxidized with a metal alkoxide inthe presence of a carbonyl compound as a hydride acceptor. Effectively the reverse of the Meerwein!Pondorff!Verley Reduction. ExamplesPhPhSOHBaMnO4, CH2Cl2OH23 CPhOSPh85% The reaction is an equilibrium process and is believed to proceed through a cyclic transition state.The use of easily reduced carbonyl compounds, such as quinone, helps drive the reaction in thedesired direction.HLR1R3MR2OLHOR4OHProposed Transition StateFirouzabadi, H.; Mostafavipoor, Z. Bull. Chem. Soc. Jpn. 1983, 56, 914!917.Djerassi, C. Org. React. 1951, 6, 207.Oppenauer, R. V. Rec. Trav. Chim. Pays-Bas 1937, 56, 137!144.OHOH3CH3COH ExamplesOHBaMnO4CH2OHCHOpivaldehyde, toluene92%H3C CH3H3C CH32 mol %F5H3CHowell, S. C.; Ley, S. V.; Mahon, M. J. Chem. Soc., Chem. Commun. 1981, 507!508.(S)-perillyl alcoholBOHF5H3C99%CH3H3CHSEMOOCH2OHCH3BaMnO4, CH2Cl2H3CHH98%OCHOHIshihara, K.; Kurihara, H.; Yamamoto, H. J. Org. Chem. 1997, 62, 5664!5665. Highly reactive zirconium alkoxide catalysts undergo rapid ligand exchange and can be used insubstoichiometric quantities.SEMOCH3CH3cat. Zr(O-t-Bu)4, Cl3CHO, CH2Cl2Burke, S. D.; Piscopio, A. D.; Kort, M. E.; Matulenko, M. A.; Parker, M. H.; Armistead, D. M.;Shankaran, K. J. Org. Chem. 1994, 59, 332!347.OHH3CCH33 Å MS86%OH3CCH3mentholKrohn, K.; Knauer, B.; Kupke, J.; Seebach, D.; Beck, A. K.; Hayakawa, M. Synthesis 1996, 1341!1344.Mark G. Charest, Jonathan William Medley9
MyersChem 115OxidationChromium (VI) Oxidants ReviewsCollins Reagent: CrO3 pyr2Ley, S. V.; Madin, A. In Comprehensive Organic Synthesis, Trost, B. M.; Fleming, I., Eds.,Pergamon Press: New York, 1991, Vol. 7, p. 251!289. CrO3 pyr2 is a hygroscopic red solid which is easily hydrolyzed to the yellow dipyridiniumdichromate ([Cr2O7]–2(pyrH )2).Luzzio, F. A. Organic Reactions 1998, 53, 1!122. Typically, 6 equiv of oxidant in a chlorinated solvent leads to rapid and clean oxidation ofalcohols. The mechanism of chromic acid-mediated oxidation has been extensively studied and iscommonly used as a model for other chromium-mediated oxidations. Caution: Collins reagent should be prepared by the portionwise addition of solid CrO3 topyridine. Addition of pyridine to solid CrO3 can lead to a violent reaction.R2CHOH HCrO4– H R2C O CrO3HCollins, J. C.; Hess, W. W.; Frank, F. J. Tetrahedron Lett. 1968, 30, 3363!3366.R2CHOCrO3H H2OR2C OCollins, J. C.; Hess, W. W.; Org. Synth. 1972, 52, 5!9. In situ preparation of the reagent circumvents the difficulty and danger of preparing the purecomplex.OHOH3CH3CCrO3, pyr, CH2Cl2 HCrO3– BH HBHolloway, F.; Cohen, M.; Westheimer, F. H. J. Am. Chem. Soc. 1951, 73, 65!68.HH3C A competing pathway involving free-radical intermediates has been identified.CH395%R2CHOH Cr(IV)R2COH Cr(III) H Ratcliffe, R.; Rodehorst, R. J. Org. Chem. 1970, 35, 4000!4003.R2COH Cr(VI)R2C O Cr(V) H ExamplesR2CHOH Cr(V)R2C O Cr(III) 2H H3CCH3 NHBocOHPhCHO OTBS(CH3)3 Tertiary allylic alcohols are known to undergo oxidative transposition.OHCr OOOCrO3HOO2. Collins ReagentOCH3CH3OCH2Cl281% overallCH3CH3( )-periplanone BStill, W. C. J. Am. Chem. Soc. 1979, 101, 2493!2495.OCH3OHCH3O2C1. H2, 10% Pd-COCH3 CH383%H 99.5% ee1. n-Bu4N F–, THFC Doyle, M.; Swedo, R. J.; Rocek, J. J. Am. Chem. Soc. 1973, 95, 8352!8357.OCH3 NHBocORittle, K. E.; Homnick, C. F.; Ponticello, G. S.; Evans, B. E. J. Org. Chem. 1982, 47, 3016!3018.O–Cr(III)CH350-g scale Fragmentation has been observed with substrates that can form stabilized radicals.HPh C O Cr(IV)(CH3)3CH3C67% 99.5% eeWiberg, K. B.; Szeimies, G. J. Am. Chem. Soc. 1973, 96, 1889!1892.CrO3, pyr, CH2Cl2!10 CWiberg, K. B.; Mukherjee, S. K. J. Am. Chem. Soc. 1973, 96, 1884!1888.HH3C2. Collins ReagentCH2Cl2CH3O2COCH3CHOCH3 CH390% overall( )-monensinDauben, W. G.; Michno, D. M. J. Org. Chem. 1977, 42, 682!685.Collum, D. B.; McDonald, J. H.; Still, W. C. J. Am. Chem. Soc. 1980, 102, 2117!2120.Mark G. Charest, Jonathan William Medley10
MyersChem 115OxidationPyridinium Chlorochromate (PCC, Corey's Reagent)Sodium Hypochlorite: NaOCl Sodium hypochlorite in acetic acid solution selectively oxidizes secondary alcohols to ketones inthe presence of primary alcohols.ClCrO3– N A modified procedure employs calcium hypochlorite, a stable and easily handled solidhypochlorite oxidant.HPCC Examples: PCC is an air-stable yellow solid which is not very hygroscopic.OH Typically, alcohols are oxidized rapidly and cleanly by 1.5 equivalents of PCC as a solution inN,N-dimethylformamide (DMF) or a suspension in chlorinated solvents.OHCH3CH3NaOCl, AcOH The slightly acidic character of the reagent can be moderated by buffering the reaction mixturewith powdered sodium acetate.H3CCorey, E. J.; Suggs, J. W. Tetrahedron Lett. 1975, 26, 2647!2650.OH91%H3CO Addition of molecular sieves can accelerate the rate of reaction.Antonakis, K.; Egron, M. J.; Herscovici, J. J. Chem. Soc., Perkin Trans. I 1982, 1967!1973.Stevens, R. V.; Chapman, K. T.; Stubbs, C. A.; Tam, W. W.; Albizati, K. F. Tetrahedron Lett. 1982,23, 4647!4650. ExamplesNwaukwa, S. O.; Keehn, P. M. Tetrahedron Lett. 1982, 23, 35!38.OHClCH3OCH3HOOPCC, 25 COTIPSHCl4Å . MOMCl, DIEAKende, A. S.; Smalley, T. L., Jr.; Huang, H. J. Am. Chem. Soc. 1999, 121, 7431!7432.PCC, CH2Cl2SH3C93%Corey, E. J.; Wu, Y.-J. J. Am. Chem. Soc. 1993, 115, 8871!8872.CH3NO1. NaOCl, AcOHO71%ONaOCl, AcOHSHH3COHHH3C86%OHBrowne, E. J. Aust. J. Chem. 1985, 38, 756!776. Treatment of tertiary allylic alcohols with PCC affords enone products via oxidative transposition.H3C CH3H3C CH3H3C CH3H3C CH3PCC, CH2Cl2CHOH 3OCrO2OCH3OCrO3CH3OCH3n-C9H19 CH2OHn-C9H19 CH2OHOH23 C94%Corey, E. J.; Lazerwith, S. E. J. Am. Chem. Soc. 1998, 120, 12777!12782.CH3NaOCl, AcOHOCH371%Winter, E.; Hoppe, D. Tetrahedron 1998, 54, 10329!10338.Dauben, W. G.; Michno, D. M. J. Org. Chem. 1977, 42, 682!685.Mark G. Charest, Jonathan William Medley11
MyersChem 115OxidationSelective Oxidations Using N-Bromosuccinimide (NBS) or Bromine NBS in aqueous dimethoxyethane selectively oxidizes secondary alcohols in the presence ofprimary alcohols.Selective Oxidations using Other Methods Cerium (IV) complexes catalyze the selective oxidation of secondary alcohols in the presence ofprimary alcohols and a stoichiometric oxidant such as sodium bromate (NaBrO3). Examples:Tomioka, H.; Oshima, K.; Noxaki, H. Tetrahedron Lett. 1982, 23, 539!542.CH3HOOHNBS, DME, H2OCH3H3CCH3HO In the following example, catalytic tetrahydrogen cerium (IV) tetrakissulfate and stoichiometricpotassium bromate in aqueous acetonitrile was found to selectively oxidize the secondaryalcohol in the substrate whereas NaOCl with acetic acid and NBS failed to give the desiredimide.OOOOOOOCH3 98%H3CCH3CH3NPhOHCH2OHCorey, E. J.; Ishiguro, M. Tetrahedron Lett. 1979, 20, 2745!2748. Bromine has been employed for the selective oxidation of activated alcohols. In the followingexample, a lactol is oxidized selectively in the presence of two secondary alcohols.OOOHO HOOH3CHOOOOBr2, AcOHHt-BuOHO HHO HHHOO 51%NPhOCH2OH7 : 3 CH3CN, H2O, 80 C48%OOCH3( )-palasoninRydberg, D. B.; Meinwald, J. Tetrahedron Lett. 1996, 37, 1129!1132. TEMPO catalyzes the selective oxidation of primary alcohols to aldehydes in a biphasic mixtureof dichloromethane and aqueous buffer (pH 8.6) in the presence of N-chlorosuccinimide (NCS)as a stoichiometric oxidant and tetrabutylammonium chloride (Bu4N Cl–).OOOH 3CNaOAcCe(SO4)2 2H2SO4, KBrO3OHHt-BuOHTEMPO, NCS,Bu4OHO HON Cl– CH2Cl2, H2O,OHCHOpH 8.677%( )-ginkgolide B0.50%Einhorn, J.; Einhorn, C.; Ratajczak, F.; Pierre, J.-L. J. Org. Chem. 1996, 61, 7452!7454.Crimmins, M. T.; Pace, J. M.; Nantermet, P. G.; Kim-Meade, A. S.; Thomas, J. B.; Watterson, S.H.; Wagman, A. S. J. Am. Chem. Soc. 2000, 122, 8453!8463. Molybdenum catalysts and H2O2 have been used to oxidize secondary alcohols in the presenceof primary alcohols:(NH4)6Mo7O24 4H2OH2O2,nBu4NCl Stannylene acetals are oxidized in preference to alcohols in the presence of bromine:OHCbzCH3 OHNH3C OOHO OOOSnBuCH3NCbzCbzBr2Bu3SnOCH370%CH3 OHNH3C OOOHOH88%Trost, B. M.; Masuyama, Y. Tetrahedron Lett. 1984, 25, 173!176.CH3NCbzOHO OOTHF, 23 COHH2Pd/C90%BuHanessian, S.; Roy, R. J. Am. Chem. Soc. 1979, 101, 5839!5841.H 3CHNOHHOHOHOON H HO OHH3C( )-spectinomycinCH3 Schreiner's thiourea has been shown to catalyze the selective oxidation of secondary alcohols byNBS:HOCF3CF3CH3OSOHF3CNNHH(0.1 equiv)NBS, CH2Cl2CF3!30 CCH3OH80%Tripathi, C. B.; Mukherjee, S. J. Org. Chem. 2012, 77, 1592!1598.Mark G. Charest, Jonathan William Medley12
MyersChem 115OxidationOOHRRAldehyde1. (CF3CO2)2IPh,ClOHOHClCH3CN, H2O, 0 COH2. NaClO2, NaH2PO4Acid2-methyl-2-butene,Sodium Chlorite: NaClO2SOTBDPSSt-BuOH, H2O Sodium chlorite is a mild, inexpensive, and selective reagent for the oxidation of aldehydes tothe corresponding carboxylic acids under ambient reaction conditions.CO2HOTBDPS82% 2-methyl-2-butene is often incorporated as an additive and has been proposed to function asa scavenger of any electrophilic chlorine species generated in the reaction.Lindgren, B. O.; Nilsson, T. Acta. Chem. Scand. 1973, 27, 888!890.ClBrKraus, G. A.; Roth, B. J. Org. Chem. 1980, 45, 4825!4830.OH3C ExamplesHH3CNaClO2, NaH2PO4,O2-methyl-2-buteneCHOTBSOHH3C( )-obtusenyneOt-BuOH, H2OCO2HTBSOCH3CH3Fujiwara, K.; Awakura, D.; Tsunashima, M.; Nakamura, A.; Honma, T.; Murai, A. J. Org. Chem.1999, 64, 2616!2617. The two-step oxidation of an alcohol to the corresponding carboxylic acid is most common.80%n-Bu3SnKraus, G. A.; Roth, B. J. Org. Chem. 1980, 45, 4825-4830.CF3OCOOO1. NaClO2, NaH2PO4,2-methyl-2-butenet-BuOH, H2OTBSOH3C CHO CO CH232. CF3CH2OH,CH3OTBSOH3CTHF, t-BuOH, H2OOHOOH 95%Nicolaou, K. C.; Ohshima, T.; Murphy, F.; Barluenga, S.; Xu, J.; Winssinger, N. J. Chem. Soc.,Chem. Commun. 1999, 809!810.OMOMOCorey, E. J.; Myers, A. G. J. Am. Chem. Soc. 1985, 107, 5574!5576.HOH3CO( )-antheridic acidOCH3OTfHOMOMNaClO2, NaH2PO4,2-methyl-2-butene90%HOCH3HCH3OOOH3C HH3CHH3 COH HOHosoya, T.; Takashiro, E.; Matsumoto, T.; Suzuki, K. J. Am. Chem. Soc. 1994, 116, 1004-1015.OOCH3OSEM1. DMP, CH2Cl2, pyr2. NaClO2, NaH2PO42-methyl-2-butene,t-BuOH, H2O98%OMOMOHOMOMCH33. CH2N2OCH3OTfacetone, H2OOH3CH3CCH3OCO2HOH . NaClO2, NaH2PO4OH3Cn-Bu3Sn1. TPAP, NMO, CH2Cl2( )-monensin AH3CCH3OCH3O2CHCH3 CH3OOOH3C HH3CH3CHH3 COH HCH3OOCH3OSEMIreland, R. E.; Meissner, R. S.; Rizzacasa, M. A. J. Am. Chem. Soc. 1993, 115, 7166!7172.Mark G. Charest13
MyersChem 115OxidationPotassium Permanganate: KMnO4Review:Fatiadi, A. J. Synthesis 1987, 85!127. In the following example, a number of other oxidants (including Jones reagent, NaOCl, and RuO2)failed:1. KMnO4, NaH2PO4, Potassium permanganate is a mild reagent for the oxidation of aldehydes to the correspondingcarboxylic acids over a relatively large pH range. Alcohols, alkenes, and other functional groupsare also oxidized by potassium permanganate.TsNNTsH Oxidation occurs through a coordinated permanganate intermediate by hydrogen atomabstraction or hydride transfer.t-BuOH, H2O, 0 CHOHTsNNTsCH3O2. (CH3)3SiCHN2HO80%HFreeman, F.; Lin, D. K.; Moore, G. R. J. Org. Chem. 1982, 47, 56!59.Rankin, K. N.; Liu, Q.; Henrdy, J.; Yee, H.; Noureldin, N. A.; Lee, D. G. Tetrahedron Lett. 1998, 39,1095!1098. Potassium permanganate
In Advanced Organic Chemistry Part B, Plenum Press: New York, 1990, p. 615!664. Carruthers, W. In Some Modern Methods of Organic Synthesis 3rd Ed., Cambridge University Press: Cambridge, UK, 1987, p. 344!410. Mark G. Charest, Jonathan William Medley Oxidation Chem 115 The notion of oxidation state is