Transcription
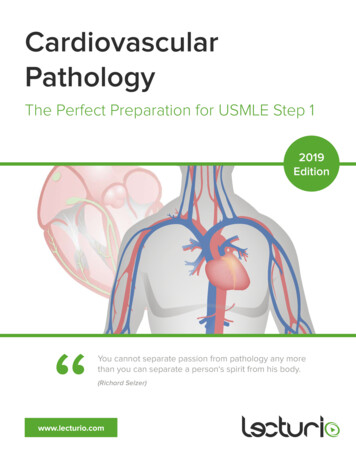
Int. J. Biol. Sci. 2022, Vol. 18IvyspringInternational Publisher386International Journal of Biological Sciences2022; 18(1): 386-408. doi: 10.7150/ijbs.65911ReviewCOVID-19: systemic pathology and its implications fortherapyQi Shen1,2, Jie Li1,2, Zhan Zhang3,4, Shuang Guo5, Qiuhong Wang6, Xiaorui An1,2, Haocai Chang1,2 1.2.3.4.5.6.MOE Key Laboratory of Laser Life Science & Institute of Laser Life Science, College of Biophotonics, South China Normal University, Guangzhou 510631,China.Guangdong Provincial Key Laboratory of Laser Life Science, College of Biophotonics, South China Normal University, Guangzhou 510631, China.Department of Neurology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou 510120, China.Guangdong Province Key Laboratory of Brain Function and Disease, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou 510120, China.Dermatology Hospital, Southern Medical University, Guangzhou 510091, China.Qilu Cell Therapy Technology Co., Ltd, Jinan 250000, China. Corresponding author: changhc@scnu.edu.cn, College of Biophotonics, South China Normal University, 55 West Zhongshan Avenue, Tianhe District,Guangzhou 510631, China. Tel: 86-20-85210089; Fax: 86-20-85216052 The author(s). This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).See http://ivyspring.com/terms for full terms and conditions.Received: 2021.08.09; Accepted: 2021.11.04; Published: 2022.01.01AbstractResponding to the coronavirus disease 2019 (COVID-19) pandemic has been an unexpected andunprecedented global challenge for humanity in this century. During this crisis, specialists from thelaboratories and frontline clinical personnel have made great efforts to prevent and treat COVID-19 byrevealing the molecular biological characteristics and epidemic characteristics of the severe acuterespiratory syndrome coronavirus 2 (SARS-CoV-2). Currently, SARS-CoV-2 has severe consequencesfor public health, including human respiratory system, immune system, blood circulation system, nervoussystem, motor system, urinary system, reproductive system and digestive system. In the review, wesummarize the physiological and pathological damage of SARS-CoV-2 to these systems and its molecularmechanisms followed by clinical manifestation. Concurrently, the prevention and treatment strategies ofCOVID-19 will be discussed in preclinical and clinical studies. With constantly unfolding and expandingscientific understanding about COVID-19, the updated information can help applied researchersunderstand the disease to build potential antiviral drugs or vaccines, and formulate creative therapeuticideas for combating COVID-19 at speed.Key words: SARS-CoV-2, immune system, nervous system, reproductive system, motor system, immunotherapy1. IntroductionCoronavirus disease 2019 (COVID-19) with acuterespiratory disease and potentially severe pneumoniahas resulted in unimaginable consequence to publichealth and loss of human life due to severe acuterespiratory syndrome coronavirus 2 (SARS-CoV-2).As the end of 5 November 2021, there have been248,467,363 confirmed cases of COVID-19, including5,027,183 deaths (https://covid19.who.int/). Thefocus of global efforts is to develop safe and effectivevaccines against COVID-19, and some vaccines areprovisionally licensed for COVID-19 prevention.However, the population of COVID-19 patients is stillsurging in the case of public vaccination.As reported, SARS-CoV-2 is a single-strandedpositive-sense RNA virus, whose genome size variesfrom 29.8 29.9 kb [1], and whose genome encodes 4structural proteins (spike surface glycoprotein (S),membraneprotein,envelopeproteinandnucleocapsid protein), 16 non-structural proteins(NSP1-16) and some accessory proteins (ORF3a,ORF3b, ORF6, ORF7a, ORF7b, ORF8, ORF9b andORF10) [2-4] (Fig. 1). With unremitting efforts ofscientific researchers around the world, the structureand function of these proteins have been moreunderstood. However, effective treatment includingdrug or vaccine is still not available for COVID-19, thestudy on SARS-CoV-2 still needs to be explored. Sofar, few studies have systematically investigated thehttps://www.ijbs.com
Int. J. Biol. Sci. 2022, Vol. 18physiologicalandpathologicalfeaturesofSARS-CoV-2 from a multisystem perspective (Fig. 1).In this review, the information about thecharacteristics, damage and treatment of SARS-CoV-2is summarized and updated by our limitedunderstanding. Importantly, we have tried to give afull insight into physiological effects and actionmechanisms of SARS-CoV-2 in order to find potentialtherapeutic strategies or control measures in this wayto combat the ongoing infection.2. Affect and Effect of COVID-192.1. COVID-19 on respiratory systemObviously, respiratory system is the first to bearthe brunt of SARS-CoV-2, which as a danger signalingis sent to immune system. As a receptor of S surfaceprotein on SARS-CoV-2, angiotensin convertingenzyme II (ACE2) is mainly expressed in type-2alveolar (AT2) cells (83% in average) [5] and othercertain cells such as epithelial cells, which existswidely in various tissues and organs including oralmucosa [6], airway [7], lung [8], colon [9], kidney [10]and prostate [11]. Depending on the context, lungs aredirectly under the strongest attack to cause lunginflammation and functional injury. As a result,pulmonary fibrosis occurs frequently in the infectedpatients, along with dry cough, dyspnea and fatiguesymptoms.Pulmonary fibrosis is characterized by theexcessive deposition of extracellular matrixcomponents including collagen and fibronectin,mainly originating from the persistence of fibroblastproliferation and activation. A molecular explanation387for pulmonary fibrosis is that fibroblast growth factor(FGF) and transforming growth factor (TGF), asinducers of collagen and fibronectin [12, 13], are bothhigh levels in COVID-19 patients [14, 15]. As reported,other upregulated pro-inflammatory cytokines, suchas platelet-derived growth factor (PDGF), vascularendothelial growth factor (VEGF), tumor necrosisfactor α (TNFα) and interleukin-6 (IL-6), also involvedin the process [16]. Another important aspect is thatongoing AT2 epithelial cell injury can recruitfibroblasts to fibrotic loci, and then fibroblastsdifferentiate into myofibroblasts to produceextracellular matrix proteins [16, 17].2.2. COVID-19 on immune systemAn effective immune response againstSARS-CoV-2 requires two arms of the immunesystem, the innate immune system and the adaptiveimmune system. The innate immune system, as thefirst line of defense of the immune system, isresponsible for rapidly recognizing the infection andtriggering alarms, in which a range of innate immunecells are involved. On the other hand, the adaptiveimmune system is essential for controlling andclearing the viral infection, the three fundamentalcomponents of which (CD4 T cells, CD8 T cells, Bcells) cooperate with each other to regulate theantigen-specific immune responses. Therefore,understanding the interaction between SARS-CoV-2and the immune system is of great importance gies, and vaccine design of COVID-19.Figure 1. Structure of SARS-CoV-2 and its infection on tissues and organs in eight major systems of human organism.https://www.ijbs.com
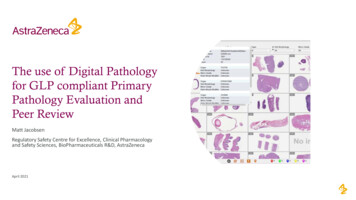
Int. J. Biol. Sci. 2022, Vol. 18388Figure 2. The immunopathology of SARS-CoV-2. SARS-CoV-2 reduced the number of monocytes, NK cells, DCs, CD4 T cells, CD8 T cells and B cells. Conversely, the virusincreased the number of neutrophils, mast cells, macrophages, memory CD4 T cells, memory CD8 T cells and memory B cells to some extent, and triggered complementsystem responses, then the host produced a strong and harmful cytokine storm, and a weak and favorable antibody response.2.2.1. Innate immune responseThe innate immune response correlates with theseverity of COVID-19, which has been proved by arange of studies [18, 19]. The innate immune systemagainst SARS-CoV-2 infection has three mainpathways: (1) limiting virus replication in infectedcells; (2) development of antiviral state in the infectionsite, including recruitment of various innate immunecells; (3) initiating the adaptive immunity [20]. All ofthese pathways require the involvement of multipletypes of innate immune cells, of which the morecommon cells are granulocytes, monocytes,macrophages and natural killer (NK) cells, but thereare also many other immune cells including differentdendritic cells (DCs), innate lymphoid cells, and mastcells.2.2.1.1. Granulocytes and monocytesA few studies have revealed that increases inmonocytes, neutrophils and eosinophils correlatewith the severity of disease in COVID-19 patients [21](Fig. 2). Liu found that the neutrophil-to-lymphocyteratio (NLR) could be served as a risk factor forearly-stage prediction of COVID-19 patients, whoseage is over 50 years old and NLR is more than 3.13 aremore likely predicted to develop critical illness [22]. Aprominent higher proportion of neutrophils andactivated mast cells was also observed in thebronchoalveolar lavage fluid (BALF) of COVID-19patients [18] (Fig. 2). The fact that chemoattractantsfor neutrophils were upregulated during COVID-19[19, 23, 24] suggested that granulocytes maysignificantly contribute to pathogenesis.CD16 monocytes were depleted in COVID-19patients and were remodeled with a phenotypic shifttoward CD14 monocytes [24]. InflammatoryHLA-DRhi CD11chi CD14 monocytes can beconsidered as a hallmark of mild COVID-19 patients[25]. Moreover, decrease of CD14lo CD16hi monocytesand the appearance of dysfunctional monocytes withlow expression of human leukocyte antigen class DR(HLA-DR) and high expression of alarming S100 wererelated to the severe COVID-19 patients [24, 25].Interestingly, Guo et al. found a unique subpopulationof monocytes, with high expression of inflammatorygenes, specifically present in the severe COVID-19cases. These inflammatory genes were potentiallyregulated by the transcription factors ATF3, NFIL3,and HIVEP2 [26]. And COVID-19 patients werereported to have greater abundance of CD14 IL-1β and IFN-activated monocytes compared to healthycontrols [27]. These data suggest the potential risk ofinflammatory cytokine storms caused by monocytes.In addition, ISGs upregulation in monocytes washeterogeneous, with no clear relevance of COVID-19severity [24].2.2.1.2. Macrophages and DCsProinflammatory monocyte-derived macrophages had higher abundance in the BALF fromsevere COVID-19 than mild cases [28]. Furtheranalysis of the heterogeneity of macrophages insevere and mild patients found that the mild patientshttps://www.ijbs.com
Int. J. Biol. Sci. 2022, Vol. 18highly expressed FABP4, while the severe patientshighly expressed FCN1 and SPP1. These data suggestthe imbalance of lung macrophage populations y macrophage microenvironment ispresent in the lungs of severe COVID-19 patients,which may contribute to recruitment of other innateimmune cells and tissue damage. Additional datashowed that macrophages in the lower airways had astronger inflammatory signature than those withinthe upper airways [29]. Conventional DCs (cDCs) andplasmacytoid DCs (pDCs) have been reported tosignificantly decrease in BALFs of patients withsevere COVID-19 [28]. Sánchez-Cerrillo et al. foundthat CD1c cDCs preferentially migrated from bloodto lungs in severe COVID-19 cases, whereas CD141 cDCs and CD123hi pDCs were depleted from bloodand were also absent in the lungs [30]. Another studyfound a decrease in the resting DCs and an increase inthe activated DCs in the lungs of COVID-19 cases [24].2.2.1.3. NK cellsAccumulating studies have reported low levelsof NK cells in the peripheral blood of COVID-19patients with moderate and severe disease [24, 31, 32].However, two reports assessing the immune cells inBALF of COVID-19 patients have revealed that NKcells are increased at this infection site [28, 29].Maucourant et al. found low numbers but a strongactivation phenotype (Ki-67 , HLA-DR , CD69 ) ofNK cells in peripheral blood of COVID-19 patientsand adaptive NK cells, with high expression ofperforin, NKG2C, and Ksp37, was increased incirculation of patients with severe COVID-19 [33].Analysis of NK cell transcriptomic signaturesfurthermore confirmed that the increased expressionof cytotoxic marker PRF1 and repair marker DDIT4 inNK cells was associated with recovered COVID-19patients [34]. In contrast, another study foundNKG2A, an inhibitory receptor, has been upregulatedon peripheral NK cells, meanwhile, the expressions ofthe activation markers CD107a, IFN-γ, IL-2, and TNFαwere decreased. These results suggest the functionalexhaustion of peripheral NK cells in COVID-19patients [31]. Moreover, in convalescent patients, thefrequency of NK cells was restored [31, 35].2.2.1.4. Complement systemThe complement system is a key component ofinnate immune response to clear pathogens andserves as a danger-sensing alarming system. Patientswith COVID-19 have been reported with theactivation of the complement system, and intensecomplement activation was related to severe disease[36]. COVID-19 patients showed the complementactivation in their lung, sera, and other organ tissue389[37, 38]. Mannose-associated serine protease 2(MASP-2) is the critical component for mediatingactivation of the lectin pathway. Nucleocapsid proteinof SARS-CoV-2 was found to interact with MASP-2[38], which led to a strong complement activation andfurther aggravated lung injury. Complementactivation products in COVID-19 patients includedthe classical/lectin (C4d), alternative (C3bBbP) andcommon pathway (C3bc, C5a, and sC5b-9), the lectinpathway recognition molecule mannose-bindinglectin (MBL). Among which, sC5b-9 and C4d weresignificantly higher in patients with respiratoryfailure than without respiratory failure [37] (Fig. 2).Similarly, complement 6 and complement factor B, asthe components of complement system, have beenreported to be elevated in serum of patients withCOVID-19, suggesting that the complement activationis an early immune response mechanism [39].2.2.1.5. Cytokine stormIt was observed that the cytokine storm wasassociated with disease severity of COVID-19. Aclinical investigation of 41 confirmed cases infectedwith SARS-CoV-2 in China revealed that the initialplasma concentrations of IL-1B, IL-1RA, IL-7, IL-8,IL-9, IL-10, basic FGF, G-CSF, GM-CSF, IFN-γ, IP10,MCP1, MIP1A, MIP1B, PDGF, TNFα, and VEGF werehigher compared to healthy individuals [21]. Furtheranalysis of both ICU and non-ICU patients found thatICU patients exhibited higher plasma concentrationsof IL-2, IL-7, IL-10, G-CSF, IP10, MCP1, MIP1A, andTNFα than non-ICU patients [21]. Similarly,additional studies quantified cytokines andchemokines in serum samples of COVID-19 patients,and the results showed remarkable increases incirculating levels of IL-6, IL-1RA, CCL2, CCL8CXCL2, CXCL8, CXCL9, and CXCL16 [19]. Thesignificant increased chemokines are likelyresponsible for the recruitment of neutrophil (CXCL1,CXCL2, CXCL6) and monocyte (CCL2 and CCL8) tothe lungs [19]. However, it is worth mentioning thatwhole blood RNA levels of cytokines were not alwaysconsistent with protein plasma levels. For example,IL-6 had not detected an increase at the transcriptionallevel in the peripheral blood of COVID-19 patients,but there is a large amount of IL-6 protein. TNFα wasonly moderately upregulated at the transcriptionallevel, while circulating TNFα was significantlyincreased [40, 41].The interferon system plays an important role inantiviral immunity, and it is critical to understand theIFN response in COVID-19. An immune analysisbased on a cohort of 50 COVID-19 patients revealedthat type I IFN response was high in mild to moderatepatients, whereas severe patients had significantlyhttps://www.ijbs.com
Int. J. Biol. Sci. 2022, Vol. 18lower type I IFN responses than mild-to-moderatepatients [41]. In contrast to severe influenza,COVID-19 patients exhibited a hyperinflammatoryprofile in their peripheral blood mononuclear cells,with a particular upregulation of TNF/IL-1β-driveninflammatory responses, and type I IFN responsescoexist with TNF/IL-1β-driven inflammation inmonocytes from severe COVID-19 patients [42]. Theseobservations were consistent with an enhancedexpression of ISGs in patients with COVID-19 [41, 43].Zhou et al. reported that the expression of 83 ISGs wassignificantly upregulated in COVID-19 patients,including IFITMs with direct antiviral activity,compared with community-acquired pneumoniapatients [18]. However, in in vitro infection modelusing cell lines, SARS-CoV-2 only induced low levelsof type I and type II IFNs, as well as moderate levelsof ISGs and a distinct proinflammatory cytokineprofiles including IL-1B, IL-6 and TNF, and manychemokines (CCL20, CXCL1, CXCL2, CXCL3, CXCL5,CXCL6 and CXCL16) [19, 40], resulting in efficientlyrestricting SARS-CoV-2 infection. A longitudinalimmunological analysis of COVID-19 revealed thatIFNα and IFNλ in moderate patients were high levelsduring the early phase and then declined, whereasIFNα and IFNλ in severe patients showed acontinuous increase in overall [23]. The dynamicchanges of type I IFNs in COVID-19 patients suggestthat type I IFNs do not control the replication ofSARS-CoV-2 in vivo but are important drivers of thedisease progression.2.2.2. Adaptive immune responseThe adaptive immune response is critical forcontrolling and eliminating most viral infections.Studies of COVID-19 patients have observed thatadaptive immune response limits COVID-19 diseaseseverity and balanced CD4 T cell, CD8 T cell, andantibody responses are protective, significantlyassociated with milder disease. Understanding thequantity and function changes in the three branches(B cells, CD4 T cells and CD8 T cells) of adaptiveimmune system in COVID-19 patients will provideinsights into immunity and pathogenesis ofSARS-CoV-2 infection, and the same knowledge alsocontributes to the vaccine development andevaluation of candidate vaccines.2.2.2.1. LymphopeniaLymphopenia is prevalent in patients withCOVID-19 and is correlated with increased diseaseseverity. Patients who suffered COVID-19 showedlower total blood lymphocyte compared to healthyindividuals, including a significant decrease in countsof CD4 T cells, CD8 T cells, NK cells and NKT cells390[23]. The lymphocyte percentages were mostly lowerthan 20% in severe patients, who were more likely toexhibit lymphopenia than mild/moderate patients[44]. Moreover, further analysis revealed that patientswith severe COVID-19 had a remarkable decrease in Tcells counts, but not B cells, especially CD8 T cellscompared with moderate patients. These clinical datasuggest that lymphopenia can be used as one of theeffective indicators of disease severity and prognosticevaluation in COVID-19 patients.2.2.2.2. CD4 T cific CD8 and CD4 T cells existed in70% and 100% of convalescent COVID-19 patientsrespectively [45], indicating that almost allSARS-CoV-2 infections elicit the T cell response,especially CD4 T cell responses. Structural proteins(spike, membrane, and nucleocapsid) of SARS-CoV-2are the prominent targets of SARS-CoV-2-specificCD4 T cells. Additional CD4 T cell responses alsoagainst NSP3, NSP4, open reading frame (ORF) 3a,and ORF8 [45]. SARS-CoV-2-specific CD4 T cells canbe detected as early as 2-4 days after the onset ofsymptoms [46, 47], which occurred in mild COVID-19patients, accelerating viral clearance. Conversely, thedelayed appearance of SARS-CoV-2-specific CD4 Tcells was associated with severe or fatal COVID-19 ( 22 days after the onset of symptoms in some cases)[46, 47]. Moreover, SARS-CoV-2-specific CD4 T cellsin severe COVID-19 patients displayed low antigenavidity and clonality [48].Virus-specific CD4 T cells have the capacity fordifferentiation into multiple helper and effector celltypes in response to SARS-CoV-2, which recruitinnate cells and provide help to B cells and CD8 Tcells, with the abilities of direct antiviral activities andfacilitating tissue repair. Studies have been revealedthat the SARS-CoV-2-specific CD4 T cells from bothacute and convalescent COVID-19 patients mainlyproduced IFN-γ, TNF and IL-2, the classical cytokinessignature during type I T helper (Th1) cell responses,with direct antiviral functions [45, 46, 49]. Anotherbranch of CD4 T cells in immune responses againstSARS-CoV-2 is the differentiated T follicular helpercells (Tfh), whose primary function is to assist B cellsin proliferation and neutralizing antibody production,participating in humoral immunity. It has been foundthat SARS-CoV-2-specific circulating Tfh cells (cTfh)were a substantial fraction of the SARS-CoV-2-specificCD4 T cells in acute and convalescent COVID-19patients [46]. And, the higher cell frequencies ofSARS-CoV-2-specific cTfh have been associated withlower disease severity [46]. Cytotoxic T helper cells(CD4-CTLs) are related cells with direct cytotoxichttps://www.ijbs.com
Int. J. Biol. Sci. 2022, Vol. 18activity. Besides cytotoxicity-associated transcripts,the SARS-CoV-2-specific CD4-CTL were highlyenriched for transcripts encoding for a range ofchemokines such as CCL3, CCL4 and XCL1. Thesechemokines participate in the recruitment of myeloidcells (neutrophils, monocytes, and macrophages), NKcells, and DCs to the sites of viral infection [50].According to the relevant data, the proportion ofCD4-CTLs and antigen-specific regulatory T cells(Tregs) exhibited an obvious negative correlation [51].2.2.2.3. CD8 T cellsCD8 T cells are important in clearing viralinfections due to their capacity for killing infectedcells. In COVID-19 patients, the presence ofSARS-CoV-2-specific CD8 T cells has been related toless severe disease [46]. Similarly to SARS-CoV2-specific CD4 T cells, SARS-CoV-2 CD8 T cells arespecific for multiple SARS-CoV-2 antigens, such asspike, nucleocapsid, membrane and ORF3a protein[45]. SARS-CoV-2-specific CD8 T cells can bedetected as early as day 1 post-symptom onset [52].The data showed that SARS-CoV-2-specific CD8 Tcells were detected in 87% of convalescent COVID-19cases but only in 53% of acute cases [46]. In the acuteCOVID-19 cases, SARS-CoV-2-specific CD8 T cellswere characterized by activation marker (CD38,HLA-DR, and Ki-67) and predominantly expressedIFN-γ, granzyme B, perforin, and CD107a, withcytotoxic effector functions [45, 53]. However,SARS-CoV-2-specific CD8 T cells in convalescentCOVID-19 patients tended to an early differentiatedmemory phenotype (CCR7 CD127 CD45RA-/ TCF1 ) [54]. From the foregoing, increasing thenumber of CD4 and CD8 T cells through thecombination of other therapeutic approaches may be abetter method for the treatment of COVID-19 patients,including the use of drug (such as CD3 antibody andCD28 antibody [55]) and drug-free (such asphototherapy [56]) therapeutic strategies.2.2.2.4. Antibody responseThe SARS-CoV-2 infected individuals exhibitedseroconversion within 20 days after symptom onset[24, 57, 58]. Seroconversion for immunoglobulin (Ig)M and IgG antibodies against SARS-CoV-2 occurredsimultaneously or sequentially [57]. A study based on173 COVID-19 patients revealed that theseroconversion rates for total antibodies, IgM, andIgG were 93.1%, 82.7%, and 64.7%, respectively [59].The spike and nucleocapsid of SARS-CoV-2 areprimary antigens testing for seroconversion [60].Spike is the main target of neutralizing antibodiesagainst SARS-CoV-2, and its receptor binding domain(RBD) is the target of more than 90% of neutralizingantibodies in COVID-19 patients [60, 61], whereas391some other neutralizing antibodies instead target theNTD [61]. Moreover, most COVID-19 patients haddetectable IgG and IgA responses to spike andnucleocapsid, whereas IgM responses were limited tospike and undetectable for nucleocapsid [60].Among most SARS-CoV-2-infected patients,neutralizing antibodies developed rapidly. Zhao’sdata showed that the antibodies were less than 40%within 1 week since onset, and could rapidly increaseto 100.0% (total antibodies), 94.3% (IgM), and 79.8%(IgG) within 15 days [59]. And neutralizing antibodiestiters stayed relatively stable for at least 5 months [62].Additionally, it is worth noting that SARS-CoV-2neutralizing antibody titers are positively correlatedwith COVID-19 disease severity [57, 60, 63].Consistently, most convalescent cases who recoverfrom COVID-19 do not exhibit high levels ofneutralizing antibodies activity. These antibodies hadlittle somatic hypermutation and were highlyenriched for the usage of gene VH1-69, VH3-30-3, andVH1-24 [61]. Furthermore, the production ofSARS-CoV-2 neutralizing antibodies did not requireaffinity maturation. Collectively, these data suggestthat the neutralizing antibodies against SARS-CoV-2are relatively easy to generate.2.2.2.5. Immune memoryImmunological memory is composed of memoryCD4 T cells, memory CD8 T cells and memory Bcells. All three major types of immune memory aresubstantially generated after SARS-CoV-2 infection.About 95% of individuals kept immune memory at 6months after SARS-CoV-2 infection [64].About 90% of subjects are seropositive forSARS-CoV-2 neutralizing antibodies at 6 to 8 monthsafter symptom onset [64]. SARS-CoV-2 RBD IgM andIgG titers steadily decreased within 1 to 6 monthsafter infection, whereas IgA was less declinedrelatively [65]. One cross-sectional analysis ofCOVID-19 subjects has revealed that SARS-CoV-2specific memory B cells were detectable in all subjectsat 6 months post-infection. Moreover, the frequenciesof memory B cells specific to spike, RBD, andnucleocapsid increased over time in the following 4 to5 months post-infection and then plateaued [64]. RBDmemory B cells had undergone affinity maturationafter infection 6 months and expressed the ontinuous evolution of humoral immunity [65].The memory CD4 T cells predominantlyconsists of Th1 and Tfh cells [64, 66]. The majority ofSARS-CoV-2 memory CD8 T cells were mainlyterminally differentiated effector memory cells(TEMRA), with small populations of central memory(TCM) and effector memory (TEM) [64]. A few studieshttps://www.ijbs.com
Int. J. Biol. Sci. 2022, Vol. 18have assessed memory CD4 T cells and CD8 T cellsat least 6 months after infection. One study found that 90% of subjects were positive for SARS-CoV-2memory CD4 T cells and 50% were positive formemory CD8 T cells after 6 months primary infection[64]. There was a steady decline in SARS-CoV-2memory CD8 T cells and CD4 T cells within 8months after infection.2.3. COVID-19 on blood circulation system2.3.1. Hematologic Parameters of COVID -19Patients with COVID-19 usually present withabnormal hematologic changes, including openia. Based on the clinical data of 1099COVID-19 cases provided by Guan et al. [67], the vastmajority of patients on admission presented withlymphocytopenia (83.2%), whereas 36.2% hadthrombocytopenia, and 33.7% showed leukopenia.And these hematological abnormalities were moreprominent in severe versus non-severe cases.Several factors may contribute to COVID-19associated lymphopenia. On the one hand, previousstudy showed that lymphocytes expressed the ACE2receptor on their surface [6], thus SARS-CoV-2directly infected those cells and eventually led to celllysis. On the other hand, the virus particlesdisseminate through the respiratory mucosa andinfect other cells, inducing a cytokine storm in thebody. It is characterized by markedly increased levelsof interleukins (IL-2, IL-6, IL-8), G-CSF, IFN-γinducible protein (IP-10) and TNFα, which promotelymphocyte apoptosis [68-70]. Furthermore, massivecytokine activation may be also associated withatrophy of lymphoid organs, including the spleen,and further impairs lymphocyte turnover [71].2.3.2. COVID-19 and cardiovascular systemPatients with prior cardiovascular disease are athigher risk for adverse events from COVID-19.Individuals without a history of cardiovasculardisease are at risk for incident cardiovascularcomplications [72]. The vast majority of COVID-19patients with previous cardiovascular luding hypertension (40%) [73, 74], whereas 10%had coronary heart disease, 17% had cardiacarrhythmias, and 4% showed heart failure [22, 75].Patients with more severe clinical presentationspresent with comorbidities such as hypertension(58%), heart disease (25%), and arrhythmias (44%) [76,77]. Additionally, cardiovascular manifestations,during COVID-19, are mostly represented by acutecardiac injury (ACI), defined as a rise of cardiactroponin values, with or without ejection fraction392decline or electrocardiographic abnormalities. Theprevalence of acute cardiac injury among COVID-19patients was 10-23% [21].Furthermore, COVID-19 patients are also at anincreased risk of venous thromboembolism and theirmain coagulation parameters (elevated D-Dimerlevels, fibrin degradation products) are also altered,especially in patients with severe manifestations [72].The direct effects of COVID-19 or the indirect effectsof infection, such as severe illness and hypoxia, maypredispose patients to thrombotic events. Preliminaryreports suggest that hemostatic abnormalities,including disseminated intravascular coagulation(DIC), occur in patients affected by SARS-CoV-2 [78,79]. Additionally, the severe inflammatory response,critical illness, and underlying traditional risk factorsmay all predispose to thrombotic events [79, 80].Overall, patients with cardiovascular diseaserepresent more than 20% of all fatal cases, with a casefatality rate of 10.5% [75].2.4. COVID-19 on nervous systemSimilar to other coronaviruses (mainlySARS-CoV-1, MERS-CoV and OC43), the possibilityof SARS-CoV-2 invading the central nervous systemhas bee
Review COVID-19: systemic pathology and its implications for therapy Qi Shen1,2, Jie Li1,2, Zhan 5Zhang3,4, Shuang Guo , Qiuhong Wang6, Xiaorui An1,2, Haocai Chang1,2 1. MOE Key Laboratory of Laser Life Science & Institute of Laser Life Science, College of Biophotonics, S