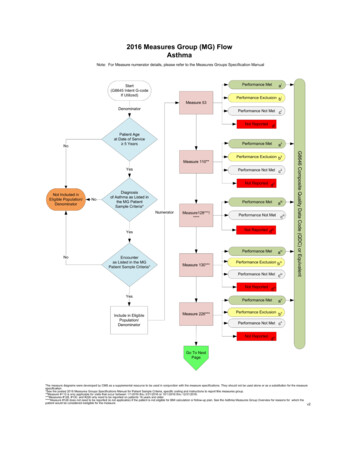
Transcription
The New VTE MeasuresKathy Phipps, RN, BSNMarch 14, 2013
VTE Initial Patient Population VTE measure set: unique in having three distinctInitial Patient Populations (or sub-populations)within the measure set, each identified by aspecific group of diagnosis codes, or lack thereof. All three sub-populations include inpatientdischarges who are 18 years of age andhave a Length of Stay (LOS) 120 days.2
VTE Initial Patient Population—SamplingThe three sub-populations must be sampled independentlyfrom each other and have their own sampling requirements. Sub-population 1 and Sub-population 2can be sampled Sub-population 3 cannot be sampled;must submit 100% of the population3
VTE Initial Patient Population—Sub-populations (slide 1 of 2) No VTE (sub-population 1) Includes patients with any ICD-9-CM diagnosis code(Principal or Other) except patients with a diagnosisof Obstetrics (Table 7.02), VTE (Table 7.03), orObstetrics-VTE (Table 7.04). If the patient has a Principal or Other ICD-9-CMdiagnosis code of Obstetrics, VTE, or Obstetrics-VTE,they are not included in the “No VTE sub-population.” Principal VTE (sub-population 2) Includes patients with an ICD-9-CM Principal diagnosisof VTE or Obstetrics-VTE4
VTE Initial Patient Population—Sub-populations (slide 2 of 2) Other VTE Only (sub-population 3) Patients with an ICD-9-CM Other diagnosis codeof VTE or Obstetrics-VTE5
VTE Initial Patient Population OverviewVTEPatients 18 years of age with a LOS 120 days and any ICD-9-CM Diagnosis Code6
VTE Initial Patient Population—Population Inclusions/ExclusionsVTE MeasuresVTE Measure Information Forms can be located in the Specifications Manual onQualityNet by selecting VTE (direct ?c Page&pagename QnetPublic%2FPage%2FQnetTier4&cid 12287673634667
VTE MeasuresVTE-1 Venous Thromboembolism ProphylaxisVTE-2 Intensive Care Unit Venous ThromboembolismProphylaxisVTE-3 Venous Thromboembolism Patients withAnticoagulation Overlap Therapy8
VTE Measures (continued)VTE-4 Venous Thromboembolism Patients ReceivingUnfractionated Heparin with Dosages/Platelet CountMonitoring by Protocol or NomogramVTE-5 Venous Thromboembolism Warfarin TherapyDischarge InstructionsVTE-6 Hospital Acquired Potentially-Preventable VenousThromboembolism9
VTE-1Patients who received VTE prophylaxis or havedocumentation why no VTE prophylaxis was given:– The day of or the day after hospital admission– The day of or the day after surgery end date forsurgeries that start the day of or the day afterhospital admission10
VTE-2Patients who received VTE prophylaxis or havedocumentation why no VTE prophylaxis was given:– The day of or the day after ICU admission (or transfer)– The day of or the day after surgery end date forsurgeries that start the day of or the day after ICUadmission (or transfer)11
VTE-3 Assesses the number of patients diagnosed withconfirmed VTE who received an overlap ofparenteral (intravenous or subcutaneous)anticoagulation and warfarin therapy.12
VTE-4 Patients who have their IV Unfractionated Heparin(UFH) therapy dosages AND platelet countsmonitored according to defined parameters suchas a nomogram or protocol.13
VTE-5Patients with documentation that they or theircaregivers were given written discharge instructionsor other educational material about warfarin thataddressed all of the following:– Compliance issues– Dietary advice– Follow-up monitoring– Potential for adverse drug reactions and interactions14
VTE-6 Assesses the number of patients diagnosed withconfirmed VTE during hospitalization (not presentat admission) who received no VTE prophylaxisbetween hospital admission and the day beforethe VTE diagnostic testing order date. This is a reverse or negative measure—Want the numerator to be low.15
VTE-6—Measure Outcome16
ContactsKathy Phipps, RN, BSNQI Specialist kphipps@acumentra.org or 503-382-3963Cheryl PrahlProject Assistant cprahl@acumentra.org or 503-382-3970Adapted from content originally developed by Telligen.This material was prepared by Acumentra Health, Oregon’s Medicare Quality Improvement Organization, under contract with the Centers for Medicare & Medicaid Services (CMS),an agency of the U.S. Department of Health and Human Services. The contents presented do not necessarily reflect CMS policy. 10SOW-OR-QRI-13-013/14/1317
VTE Measures (continued) VTE-4 Venous Thromboembolism Patients Receiving Unfractionated Heparin with Dosages/Platelet Count Monitoring by Protocol or Nomogram VTE-5 Venous Thromboembolism Warfarin Therapy Discharge Instructions VTE-6 Hospital