Transcription
4th International Symposium on Independent Component Analysis and Blind Signal Separation (ICA2003), April 2003, Nara, JapanAPPLICATION OF INDEPENDENT COMPONENT ANALYSIS TOCHEMICAL REACTIONSS.Triadaphillou, A. J. Morris and E. B. MartinCentre for Process Analytics and Control TechnologySchool of Chemical Engineering and Advanced MaterialsUniversity of Newcastle, Newcastle upon Tyne, NE1 7RU, UKSophia.Triadaphillou@ncl.ac.uk, julian.morris@ncl.ac.uk, e.b.martin@ncl.ac.ukdetailed understanding of the reaction mechanisms.Thus by-products may be produced that are unknownand information on their existence cannot bedetermined using existing spectral interpretationmethods. There is consequently a need for moreadvanced methods for the resolution of mixtures ofchemical reactions.The paper investigates a number of calibration freemethods for the resolution of mixtures. A simulateddata set from a first order reaction was generated fordifferent reaction rates. Techniques includedMultivariate Curve Resolution-Alternating LeastSquares (MCR-ALS) [2] and IndependentComponent Analysis (ICA) [3]. Specifically two ICAalgorithms were investigated, FastICA [4] and JointApproximate Diagonalization of Eigenmatrices(JADE) [5] with the results of JADE being used asan initial estimate in the MCR-ALS algorithm.Applications of ICA have previously been reportedin the areas of voice and sound separation,biomedical signal processing, financial time series,wireless communications and image featureextraction.ABSTRACTThe analysis of kinetic data monitored usingspectroscopic techniques and its resolution into itsunknown components is described. IndependentComponent Analysis (ICA) can be considered acalibration free technique with the outcome of theanalyses being the spectral profiles of the unknownspecies. This enables the realisation of qualitativeinformation concerning the identification of thenumber and type of components present within thereaction mixture over time. The ICA approaches ofFastICA and JADE and the calibration free techniqueof multivariate curve resolution-alternating leastsquares were applied to the mixture spectra of a firstorder synthetic reaction. For all approaches the signalwas successfully separated from the constituentcomponents.1.INTRODUCTIONReaction monitoring is a major challenge across theprocess industries. This form of monitoring typicallyinvolves the measurement and prediction of theconcentration of a number of components in achemical reaction and the determination of thenumber of components in the mixture. Furthermorethe qualitative information extracted from thespectral analysis is of importance in terms of processunderstanding.Traditionally calibration models are built to predictthe property of interest. However the success of theapproach depends on a number of factors.Calibration modelling is time consuming with thefinal model being sensitive to changes in processconditions. In addition it only provides quantitativeinformation about the property of interest with noinformation about side reactions and intermediates[1]. Moreover in industrial processes a number ofunknown components may be present in the mixturebecause of operational disturbances or a lack of2. DETERMINATION OF THE NUMBER OFCOMPONENTSThe first step is the estimation of the number ofcomponents in the mixture. This factor is related tothe amount of variance present as a result of sourcessuch as noise, background and baseline changes. Onemethod that has been applied for the identification ofthe number of latent variables [6] is PrincipalComponent Analysis (PCA). PCA has been widelyapplied for the decomposition of the covariancematrix. A data matrix representing I observations onJ variables can be decomposed as follows:D T VT879(1)
where D is the spectral response, (I J ) , V is theloadings matrix, ( J K ) and T is the scores matrix,( I K ). Each principal component is a linearcombination of the J variables and accounts for themain sources of variation. The current principalcomponent is mutually orthogonal to the set ofprincipal components previously calculated.Evolving Factor Analysis (EFA) [7] is an alternativeapproach to the estimation of the number ofcomponents in a mixture. It uses the idea of thesequential expanding window. A series of spectrafrom a reaction mixture are measured for differentwavelengths and these are arranged into a datamatrix. The order of the spectra provides additionalinformation on the behaviour of a chemical reaction.This is taken into account through the formation ofsub-matrices by adding rows to an initial top submatrix, top down, or by adding rows to an initialbottom sub-matrix, bottom up. The rank of thematrix increases every time a row is added and byanalysing these ranks as a function of the number ofadded rows, time widows are derived where aspecific number of principal components are present.This is the first step in EFA. The number of speciesinvolved is equal to the number of significanteigenvalues of the second moment matrix. Thus asnew absorbing species start to become significant,new factors/eigenvalues evolve. Hence as a newcomponent elutes in the overlapping peak, thepresence of an additional eigenvalue is required toexplain this variability. EFA thus takes advantage ofinformation, which is sometimes unused in the timedomain. In a reaction, the compound that appearsfirst in the spectra should also be the first todisappear.KR ( t , l) c i ( t )s i (l)(2)i 1where c i ( t ) is the concentration of component, i, attime, t, and s i (l) is the spectral response ofcomponent, i, at wavelength, l.An advantage of this method is that knowledge of theconcentration of all the compounds at the beginningof the kinetic process is not required. Tauler et al [9]developed a technique to reconstruct theconcentration profiles in reactions. For this approach,the compound windows were found by connectingthe line of the compound that first appears with theline of the last compound that appears. Both linesare combined in a single figure from which theconcentration windows can be reconstructed. Theseprofiles of the eigenvalues can be considered as afirst rough estimate of the concentration profiles.Based on the Beer Lambert law, the aim of MCRALS is the optimal decomposition of the data matrixD into the product of two smaller matrices, C , thatrelates to the concentrations and S T that denotes thespectral profiles:D CS T E(3)E is the error-related matrix. The decomposition ofdata matrix D is performed by iterative optimization.The error in the raw data set is minimised using thefollowing equations under suitable constraints for Cand S T :min D CS T(4)min D CS T(5)ST3. MULTIVARIATE CURVE RESOLUTIONALTERNATE LEAST SQUARES (MCR-ALS)CMCR-ALS is an iterative resolution methoddeveloped by Tauler. It has been applied to mixturedynamic processes that have been monitored throughspectroscopic techniques and also to other chemicaldata whose instrumental responses obey BeerLambert law. Beer’s law states that the spectralresponse of the components is independent of timeand concentration [8]. In the case of reactionmonitoring, the spectroscopic response, R, is afunction of two variables, the time, t, and the spectralwavelength, l.As a result, a mixture of Kcomponents gives a response:A description of the MCR-ALS algorithm can befound in the work of De Juan et al, [10], [11]4. INDEPENDENT COMPONENT ANALYSISAn alternative calibration free resolution method thatis considered is Independent Component Analysis(ICA). ICA can be used to identify the spectralprofile of each species in a mixture, i.e. to identifythe unknown components. ICA is a method designedto offer a solution to the Blind Source Separationproblem, i.e. separate the source signals from the880
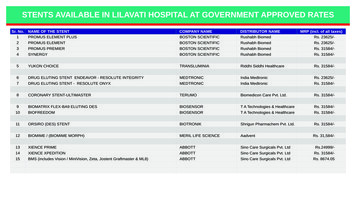
mixture observations. ICA can be considered as anextension to PCA in that while PCA finds principalcomponents that are uncorrelated and that are linearcombinations of the observed variables, ICA isdesigned to extract components that are independentand that constitute the observed variables.Basically an ICA model is a “statistical latentvariable model” in the sense that it describes how theobserved data are generated by a process of mixingthe recorded signals, s i . The signals s i arestatistically mutually independent by definition andare called independent components. The basicproblem is:d i a i1s1 a i 2s 2 a in s n i 1, , nelements of ŝ , i.e. they have non-Gaussiandistributions. Non-gaussianity can be measured byeither kurtosis or negentropy.Two ICA methodologies were evaluated, FastICAand Joint Approximate Diagonalization ofEigenmatrices (JADE). JADE is a cumulative-basedbatch algorithm for source separation. Specifically itis a method that uses higher-order cumulative tensorsthat are a generalisation of the covariance matrix. Forthis family of methods, the fourth-order cumulativetensor is used to make the fourth-order cumulantszero or as small as possible. JADE computes theeigenvalue decomposition of a symmetric matrix.5. EXPERIMENTALGENERATING THE SYNTHETIC DATA SET(6)where d i are the observed random variables that aremodeled as a linear combination of n randomvariables, s i , and a ij , i, j 1, , n are realA first-order synthetic data set was generated byconsidering a starting material A to which a reagentwas added. As a result A is converted to product Bwith a specific rate constant, k1, and B is convertedcoefficients that are assumed to be unknown. It isalso assumed that each mixture d i and eachindependent component s i are random variables andnot true time signals or time series. Equation 6 can berewritten in vector matrix notation:d AsK1(7)k1 0.8k1 0.8k1 0.8where d is a column random vector whose elementsare d i , s is a column random vector whoseelements are si and A is a matrix with elements a ij .k2 0.8k2 0.08k2 0.008Experiment 1Experiment IIExperiment IIIThe data was obtained from the following kineticequations:The statistical estimation problem concentrates ontwo aspects, first under what conditions can themodel be estimated and secondly what can beestimated. In practice both the mixing coefficients,a ij , and the independent components, s i , could be[A]i [A]0 exp( k1t i )[B]iestimated using the observed variables d i . Forsimplicity it is assumed that d is a pre-whitenedvector, i.e. all its components are uncorrelated andtheir variances are equal to unity. An alternative wayto express the ICA model is:sˆ WdK2to product C with reaction rate, k2, i.e. A B C .Several experiments were performed for differentrate constants. For the data set generated from thefirst order synthetic reaction, the reaction rates tookthe values: [A]0 k 1k 2 k1(exp( k 1 t i ) exp( k 2 t i ))[C]i [A]0 [A ]i [B]i(9)(10)(11)where [A ]0 is the initial concentration of A, and[A]i , [B]iand [C]i are the concentrations of A, Band C respectively at time point i.Fig. 2 shows the concentration profiles of the threecomponents for the reaction for experiments I, II andIII respectively. The spectral profiles for thecomponents are the same for all experiments sincethe same data was used in all three experiments andare presented in Fig. 1 (lower right hand-side).Based on the data decomposition, it is expected thatthe pure spectra of the components should be the(8)where ŝ is the estimate of s, d i is the observedrandom variables and W is a separating matrix whichhas to be estimated. W can be defined as the weightmatrix of a two-layered feed-forward network whereŝ is the output and d is the input. The network isconstrained to have statistically independent881
same for all experiments, although the concentrationprofiles in the different experiments need not have acommon shape. The varying shape of the kineticprofiles in the different experiments can be due eitherto different underlying kinetic models, differentinitial concentrations or to different reactionconstants. In this case the different reaction constantsproduce the different concentration profiles for eachexperiment.Component AComponent BComponent C0.90.80.80.70.70.60.60.50.40.50.40.30.30.20.26. RESULTS AND er creating the experimental data sets, PCA wasinitially applied for the estimation of the number ofcomponents. For the specific reaction beingconsidered, and for all three experiments, threecomponents were selected since the eigenvalue of thethird component was still in excess of unity. This isin accord with the expected result.EFA was also used to estimate the number ofcomponents and to define the reaction process. Theresults of the first experiment are plotted in Fig.3.Similar results were obtained for the other twoexperiments. It can be observed that the forwardanalysis indicates that three independent factors haveevolved. One factor appears at the onset of thereaction, a second soon after the first and a third afterthe second. It is clear that these three factorscorrespond to the reagent, the intermediate and thefinal product. The backward analysis suggests thatthere is only one factor remaining at the end of thereaction with the other two factors disappearing.Once again the results confirm what is known aboutthe reaction.The next step was to define the spectral profiles foreach of the experiments. The problem of spectralanalysis in chemical mixtures represents a verysimilar problem to that of ICA since it is assumed inspectral analysis that the components of interest arestrongly related to the data of the mixture throughBeer Lambert's law. FastICA was used for theseparation of the spectral profiles (Fig.4). ICA wasperformed with ‘tanh’ non-linearity and theindependent components were estimated in parallelby FastICA. This approach is similar to themaximum likelihood estimation for supergaussiandata. The results from the application of the JADEalgorithm can be seen in Fig. 5.10Pure component spectraConcentration profile1.41Component AComponent BComponent C0.9Component AComponent BComponent 0100120Fig. 1: Concentration profiles of A, B and C forexperiment I, II and III respectfully as defined byequations 9, 10 and 11E x p e rim e n t I1Absorbance0 .90 .80 .70 .60 .50 .40 .30 .20 .11 02 03 04 05 0W6 07 08 09 01 0 01 1 01 2 08 09 01 0 01 1 01 2 08 09 01 0 01 1 01 2 0a ve le n g t h sE x p e rime n tII10 . 90 . 8Absorbance0 . 70 . 60 . 50 . 40 . 30 . 20 . 11 02 03 04 05 0W6 07 0a ve le n g t h sE x p e rime n tIII10 . 90 . 8Absorbance0 . 70 . 60 . 50 . 40 . 30 . 20 . 11 02 03 04 05 0W(12)The matrix D is then used to resolve the spectralprofiles of each species. The aim of this paper is toshow that although different experiments areperformed, in each case the results are the same andthe components are identified successfully.1Component AComponent BComponent CConcentrationConcentrationD conc T spectraConcentration profileConcentration profile10.90The concentration and pure component spectraprofiles can be combined to produce a matrix, D thatconsists of a number of spectra for differentwavelengths. The plots of this matrix can be seen inFig. 2.6 07 0a ve le n g t h sFig. 2: Graphical representation of matrix D882
Experiment IForward Analysis20JA D Ere s u lt s - E x p e rim e n t I1 .2C om ponent BC om ponent AC om ponent C10100 .8-20absorbancesSingular Value10100 .60 .4-4010010203020405060TimeReverse Analysis7080901000 .2010Singular Value-0 .202040010JA D E60w a ve le n g t h s80100120r e s u l t s - E x p e r i m e n t II1 .2-20C om ponent CC om ponent AC om ponent B1100 .801020304050Time60708090absorbances-4010100Fig. 3: EFA plots for the first experiment. The thickline defines the noise level and the solid lines abovethe noise level show the number and possiblelocation of components appearing and disappearingduring the kinetic process.0 .60 .40 .20-0 .202040JA D E60w a ve le n g t h s80100120r e s u l t s - E x p e r i m e n t III1 .2C om ponent CC om ponent AC om ponent B10 .8F a s t IC A- E x p e rim e n t Iabsorbances420-202 04 06 08 01 0 00 .60 .41 2 040 .2200-202 04 06 08 01 0 01 2 0-0 .2420-202 04 0F a s t IC A46 0R e s u lt s8 01 0 01 2 02 04 06 08 01 0 01 2 002 04 06 08 01 0 01 2 002 04 06 08 01 0 01 2 0420-2420-2F a s t IC A R e s u l t s - E x p e r i m e n t I 20420-2420-24060w a ve le n g t h s80100120Although the results appear almost perfect, furtherimprovement can be achieved. This can be done byapplying constraints to the data. As mentioned beforeMCR-ALS is a method used for the improvement ofan initial estimate. During the procedure, the initialestimates of the concentration profiles, or of thespecies spectra, are given and new concentrationprofiles are calculated by least-squares. Normally forMCR-ALS, the results from EFA are used. Howeverin this application the results from the JADEalgorithm were used as an initial estimate of thespectral profiles. A comparison of the results of thisprocedure for the three experiments and of what wasexpected can be seen in Fig 6. A number ofconstraints such as unimodality and non-negativitywere also imposed. Once the concentration profilesand the pure spectra become stable, the resulting datamatrix can be resolved.From Fig.6 it can be observed that the results areextremely promising. In all three experiments, thespectra were resolved identically, showing that thedifferent reaction rates and mixture spectra do notinfluence the hidden information relating to theidentification of the species. Component A had an0020Fig. 5: Estimated spectral profiles from the JADEalgorithm for all the experiments- E x p e r i m e n t II2-20Fig. 4. Estimated spectral profiles by the FastICAalgorithm for all experimentsThese results indicate that ICA is very effective forthe analysis of spectral data. The difference inscaling does not affect the qualitative informationgained. The main peaks are situated where expectedand the componets are easily recognisable.883
overlap between what was expected and what wasestimated. Component’s C predicted and real valueswere almost identical. Finally component’s Bestimated peaks were situated at the samewavelengths as the real peaks have to be situatedmaking component B easily recognisable.8. ACKNOWLEDGEMENTSThe author would like to acknowledge the EPSRCaward, KNOWHOW (GR/R/938010) and the EUproject BATCHPRO (HPRN-CT-2000-0039) forfinancial support.9. REFERENCESE x p e rim e n t I10 .90 .8Vandeginste, B.G.M., Massart, D.L, Buydens,L.M.C., Jong S.De, Lewi, P.J., SmeyersVerbeke, J., Handbook of Chemometrics andQualimetrics: Part B. 1998: Elsevier.R.,MCR-ALS.2002,2. /www.ub.es/gesq/roma/roma.htm3. Hyvarinen, A., Karhunen, J., Oja, E.,Independent Component Analysis. Adaptive andLearning Systems for Signal Processing,Communications and Control, ed. S. Haykin.2001: John Willey and Sons.4. Hyvarinen, A., Oja, E., A Fast-point algorithmfor Independent Component Analysis. NeuralComputation, 1997. 9: p. 1483-14925. Cardoso, J.-F., High-order contrasts forindependent component analysis. NeuralComputation, 1999. 11(1): p. 157-1926. Miller, J.C., Miller, J.N., Statistics for AnalyticalChemistry. Third ed. 1993: Ellis Horwood Ltd.7. Keller, H.R., Massart, D.L., Evolving y Systems, 1992. 12(3): p. 209-224.8. Muller A., Steele, D., On the Extraction ofSpectra of Components from Spectra ofMixtures. A Development in Factor Theory.Spectrochimica Acta, 1990. 46(5): p. 817-842.9. Tauler, R., Barcelo, D., Multivariate CurveResolution Applied to Liquid Chromatographydiode Array Detection. Trends in AnalyticalChemistry, 1993. 12(8): p. 319-327.10. De Juan, A., Maeder, M., Martinez, M., Tauler,R., Application of a Novel Resolution ApproachCombining Soft- and Hard-modelling Featuresto Investigate Temperature-dependent KineticProcesses, Analytica Chimica Acta, 442, p. 337350, 200111. De Juan, A., Maeder, M., Martinez, M., Tauler,R., Combining Hard- and Soft-Modelling toSolve Kinetic Problems, Chemometrics andIntelligent Laboratory Systems, 54: p. 123-141,2000.1.Absorbances0 .70 .60 .50 .40 .30 .20 .101 02 03 04 05 06 07 0W a ve le n g t h s8 09 01 0 01 1 01 2 0E x p e r i m e n t II10 .90 .8Absorbances0 .70 .60 .50 .40 .30 .20 .101 02 03 04 05 0W6 07 0a ve le n g t h s8 09 01 0 01 1 01 2 0E x p e r i m e n t III10 .90 .8Absorbances0 .70 .60 .50 .40 .30 .20 .101 02 03 04 05 06 07 0W a ve le n g h t s8 09 01 0 01 1 01 2 0Fig. 6: Results of MCR-ALS with an initial estimateby JADE. Solid lines are the true profiles. Dottedlines are the estimated profiles7. CONCLUSIONSThe ability of ICA to handle component spectra wasexamined. The application of ICA to an artificiallygenerated spectral data set for different reaction rateshas demonstrated that it is an effective approach toits resolution. Both FastICA and JADE can beregarded as another method for the resolution ofchemical mixtures since they both extractedrecognizable spectra. The combination of MCR-ALSand JADE also gave good results. ICA has shownthat unknown components in a mixture can beidentified by the spectra of separated independentcomponents. A further advantage of ICA is that itenables the implementation of the resolution of datain limited time. ICA can also be applied in processmonitoring and control. This area is now underconsideration.884
The paper investigates a number of calibration free methods for the resolution of mixtures. A simulated data set from a first order reaction was generated for different reaction rates. Techniques included Multivariate Curve Resolution-Alternating Least Squares (MCR-ALS) [2] and Independent Component Analysis (ICA) [3]. Specifically two ICA