Transcription
Implementation & Documentation Requirements for ISO 22716:2007 CertificationISO 22716 is an international standard that gives guidance for the production, control, storage andshipment of cosmetic products. It deals with all aspects of the supply chain of cosmetic products. Theguidelines cover the quality and safety of the product, and they affect manufacturers, as well assuppliers, retailers, brand holders and retailers of cosmetic products. It does not, however, cover safetyaspects for the personnel engaged in the plant, or the protection of the environment.With ISO 22716 implementation using MyEasyISO software platform, not only do you receive a guidelinefor your business’ path, you also commit to ensuring the safety, quality and excellence of your product.In accordance with the ISO 22716 guide, the manufacturing system of your company will be audited andinspected on the following areas: Complaint and recallsContracting/ subcontractingDocumentation and recordsInternalauditsandLaboratory quality controlsMaterial managementPackaging and labellingPersonnel, HR & TrainingPremises,buildingsfacilities maintenanceorProduction and in-processcontrolsStorage and distributionPage 1USAUKwww.MyEasyISO.com616 - Corporate Way, Suite 2 #5321, Valley Cottage, New York 10989, USAHong KongAustraliaIndiaSaudi ArabiaUAEAfricaEurope
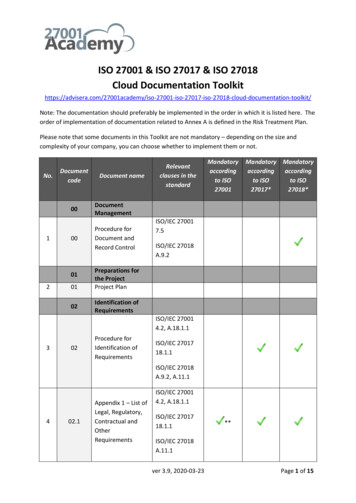
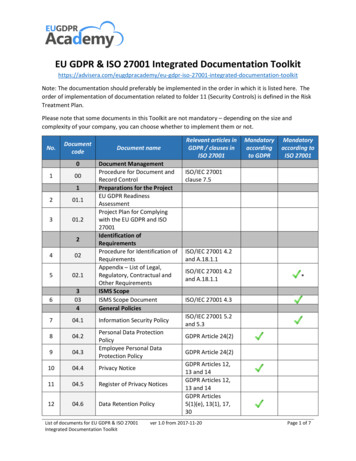
Below you ll find all the documentation that it is needed to meet ISO 22716:2007, Cosmetics GMP & howMyEasyISO can make this compliance simple, easy, employee friendly, effective and always audit ready :SectionRequirementRequired documentationDescriptionPersonnel3Initial Orientation ProgramPremises Cleaning and SanitationInspection4.11Visitors and UntrainedPersonnelPremises Cleaning essHRPremises Maintenance ProgramMaintenance4.13Pest ControlPest ControlMaintenanceEquipment Installation QualificationControl of Measuring and Test InstrumentsEquipment Cleaning and lationCalibrationEquipment Cleaning Identification and 5.35.45.6Raw Materials andPackaging6Page 2USAResponsibilities of personnelTrainingPersonnel Hygiene and HealthUKJob DescriptionsPersonnel and TrainingInitial Orientation ProgramGowning, Hand Washing and ConductResponse Plan for Incidents Involving BiohazardsMyEasyISOModuleEquipment MaintenanceEquipment Technical Data SheetEquipment Maintenance ChecklistEquipment Maintenance RequestEquipment Maintenance Work OrderEquipment Maintenance RecordPurchasing and Assessment of SuppliersSupplier AuditsSupplier Corrective Action Request (SCAR)Performance Evaluation of Suppliers ofSignificant Materials and ServicesIncoming InspectionRaw materials specificationsIdentification & Traceability of RawMaterials, Manufactured, and Packagedwww.MyEasyISO.com616 - Corporate Way, Suite 2 #5321, Valley Cottage, New York 10989, USAHong KongAustraliaIndiaSaudi urchaseQualityOperationsQualityAfricaEurope
SectionRequirementRequired 7.2.57.2.67.2.7Manufacturing operations:Availability of relevantdocuments7.2.2Start-up Checks7.3.17.3.37.3.47.3.5Packaging OperationsProduction and Process Control of Chemical Process OperationsProduction and Process Control of Machining Process OperationsOperationsProduct SpecificationsProduct Bill of Materials (BOM)Production Work OrderOperationsDocumentedWork Instructions for the use of specificInformationequipment (boiler, reactors, filters, drillers,In-Process Inspection (for chemical and machining Quality) Line Clearance ProcedureProductionDocumentedInformationProduction and Process Control of Packaging Process DocumentedInformationPackaging Product SpecificationsPage 3Finished Product8ProductionPackaging Product Bill of MaterialsPackaging Work 7.3.2Packaging Operations(continued)Start-up checksWork Instruction for the use of specific equipment (as DocumentedInformationsealers)Packaging Line Clearance ProcedureDocumentedInformation7.3.6In process ControlPackaging In-Process InspectionDocumentedInformation8.18.28.3Finished Products8.4ShipmentFinal product specificationFinal InspectionProduct ReleaseShipping n8.5ReturnsCustomer O.com616 - Corporate Way, Suite 2 #5321, Valley Cottage, New York 10989, USAHong KongAustraliaIndiaSaudi ArabiaUAEAfricaEurope
SectionRequirementRequired documentationDescriptionQuality Control Laboratory99.2Test Methods9.3Acceptance criteria9.4Results9.5Out-of-Specification Results9.6Reagents, solutions, referencestandards, culture media9.79.810OOSproduct10.1Wastes1112Reprocessed finished productsand bulk productsWastesDeviationsSubcontractingComplaints &Recalls1410.2SamplingRetain SampleRejected finished products, bulkproducts, raw materials andpackaging materialsAnalysis Procedures (for those not included inthe EU Pharmacopoeia)Raw material, in process or final productspecifications, establishing the acceptanceRaw material, in process or final testing resultsforms (indicating the acceptance criteria)OOS ProcedureQualityReceipt and Storage of ChemicalsPreparation and Standardization of SolutionsCleaning and washing of laboratory glasswareLaboratory Basic Safety RulesDocumentedInformationSampling ProcedureRetainsControl of Non-Conforming ProductNon-Conforming Event ProcedureRoot Cause Analysis (RCA) ProcedureRework ormationNon ConformityHandling, Storage, Treatment and Disposal of WasteWastesManagementSubcontractor management & ControlPurchase13.113.2DeviationsDeviation ProcedureNon Conformity14.2Product ComplaintsCustomer Complaints ManagementCustomerCompliant14.3Product RecallsRecall ProcedureNon ConformityChange ControlCreating and Changing SpecificationsChangeManagementRisk Assessment ProcedureInternal AuditChangeControl15Page 4MyEasyISOModuleUSAUKwww.MyEasyISO.com616 - Corporate Way, Suite 2 #5321, Valley Cottage, New York 10989, USAHong KongAustraliaIndiaSaudi ArabiaUAEAfricaEurope
SectionRequirementRequired documentationDescriptionInternal AuditsInternal eCorrective and Preventive Action System ProcedureNonConformityInternal Audits ProcedureInternal AuditDocument Control and Data Control ProcedureDocumentedInformationSignature Authority for Controlled DocumentsDocument Retention Storage and DispositionGood Documentation PracticesDIVERSE MANAGEMENT CONSULTANCY LTDP.O Box 5617-00100, Nairobi, Kenya Tel: 254 (0) 20 2007614 Email: info@diversemcltd.comPage 5USAUKwww.MyEasyISO.com616 - Corporate Way, Suite 2 #5321, Valley Cottage, New York 10989, USAHong KongAustraliaIndiaSaudi ArabiaUAEAfricaEurope
ISO 22716 is an international standard that gives guidance for the production, control, storage and shipment of cosmetic products. It deals with all aspects of the supply chain of cosmetic products. The guidelines cover the quality and safety of the product, and they affect manufacturers, as well as suppliers, retailers, brand holders and retailers of cosmetic products. It does not, however .