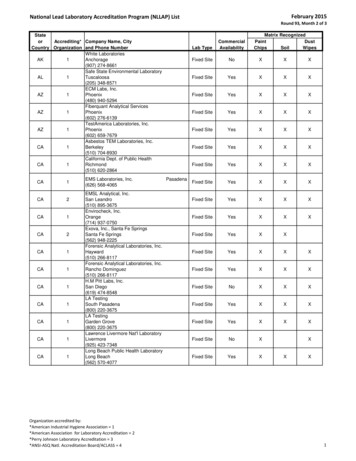
Transcription
Government of IndiaMinistry of Agriculture & Farmers Welfare(Department of Agriculture, Cooperation & Farmers Welfare)DIRECTORATE OF PLANT PROTECTION, QUARANTINE & STORAGENH IV, FARIDABAD – 121 001 (Haryana)GUIDELINES FOR ACCREDITATION OF CHEMICAL ANDBIO-PESTICIDE TESTING LABORATORIES AS PER ISO170251 Page
Necessity for accreditationAccreditation is the formal recognition, authorization and registration of a laboratory that hasdemonstrated its capability, competence and credibility to carry out the tasks it is claiming to be able todo. It provides feedback to laboratories as to whether they are performing their work in accordance withinternational criteria for technical competence.As per National Accreditation Board for Testing and Calibration Laboratories (NABL) accreditation isthe third party attestation related to a conformity assessment body (Laboratory) conveying the formaldemonstration of its competence to carry out specific conformity assessment task.In simple words, for Pesticides Testing Laboratories, accreditation is necessary to show theircompetence in the work of analysis of pesticides. Laboratory accreditation is a mean to improveStatkeholders confidence in the test reports issued by the laboratory, so that the stakeholders likePesticide manufacturers and their association may not doubt and shall accept the reports withconfidence.Benefits of AccreditationFormal recognition of competence of a laboratory by an Accreditation body in accordance withinternational criteria has many advantages like:1. Increased confidence of all stakeholders in Testing Reports issued by the laboratory.2. Better control of laboratory operations and feedback to laboratories as to whether they have soundQuality Assurance System and are technically competent3. Savings in terms of time and money due to reduction or elimination of the need for re-testing ofproducts.ISO 17025ISO (International organisation for standardisation) is an independent, non-governmental organizationwith a membership of 162 national standards bodies. Through its members, ISO brings together expertsto share knowledge and develop voluntary, consensus-based, market-relevant International Standardsthat support innovation and provide solutions to global challenges.ISO 17025, General requirements for the competence of testing and calibration laboratories, is theinternational reference for testing and calibration laboratories wanting to demonstrate their capacity todeliver reliable results.ISO 17025 enables laboratories to demonstrate that they operate competently and generate valid results,thereby promoting confidence in their work both nationally and around the world. It also helps facilitatecooperation between laboratories and other bodies by generating wider acceptance of results betweencountries. Test reports and certificates can be accepted from one country to another without the need forfurther testing, which, in turn, improves international trade.2 Page
Recognized Organization in India for providing accreditation as per ISO 17025Although there are few autonomous bodies/ institutes which are providing accreditation as per ISO17025. However, National Accreditation Board for Testing and Calibration Laboratories (NABL) whichis a constituent Board of Quality Council of India is an internationally recognized board foraccreditation, as per International standards. NABL has been established with the objective of providingGovernment, Industry Associations and Industry in general with a scheme of Conformity AssessmentBody’s accreditation which involves third-party assessment of the technical competence of testingincluding medical and calibration laboratories, proficiency testing providers and reference materialproducers.NABL maintains its linkages with the international bodies like International Laboratory AccreditationCo-operation (ILAC) and Asia Pacific Laboratory Accreditation Co-operation (APLAC). NABL is a fullmember of both and regularly takes part in the Technical Committee Meetings of both ILAC & APLAC,engaged upon development and updating of guidelines connected with accreditation activities. In orderto achieve the objective of the acceptance of test/ calibration data across the national borders, NABLoperates and is committed to update its laboratory accreditation system as per international norms.The laboratory accreditation services to testing and calibration laboratories are provided by NABL inaccordance with ISO 17025: 2017 ‘General Requirements for the Competence of Testing andCalibration LaboratoriesRequirements and procedure for getting accreditation from NABLGetting ready for accreditationIt is very important for a laboratory to make a definite plan for obtaining accreditation and nominate aresponsible person as Quality Manager, who should be familiar with the laboratory’s existing qualitysystem to co-ordinate all activities related to seeking. The laboratory should carry out thefollowing important tasks towards getting ready for accreditation:.1. Get fully acquainted with all relevant documents and understand the assessment Procedure andmethodology of making an application.2. Depute technical officers for training on Quality Management System ISO 17025, Internal audit,Measurement Uncertainty, Inter Laboratory Comparison ( ILCP) conducted by Bureau of IndianStandard (BIS) at regular intervals at different places all over the country throughout the year.Training of the personals on ISO 17025:2017 will provide the knowledge regarding followinggeneral requirements for a testing laboratory:1. General requirements(a) Impartiality(b) confidentiality2. Structural requirements3 Page
(a) Legal(b) Management2. Resource requirements(a)(b)(c)(d)(e)ManpowerFacilities and Environmental conditionsEquipmentTraceability ( Certified Reference Material and Calibration of Equipment)Product and services3. Process w of request, tenders and contractsSelection, verification and validation of methodsSamplingHandling of test itemsTechnical recordsEvaluation of measurement of uncertaintyValidity of resultsReporting of resultsComplaintsNon conforming workControl of Data and Information management4. Management system requirements(a)(b)(c)(d)(e)(f)(g)(h)Management system documentationControl of management system documentsControl of recordsAction to address risks and opportunitiesImprovementCorrective actionsInternal auditsManagement reviews3. Based on the requirement of ISO 17025, make following document:1.Quality manual – as per the guidance available in NABL document No-160 which includes:(a) Quality policy(b) Quality objectives(c) Structural requirements Legal Documents (evidence of legality) Scope of work (scope of accreditation) Organizational chart Authorizations4 Page
2.Control of recordsISO 17025 Technical/ Resource Requirements1. Personnel Procedure Trainings Training calendar Training report Technical training effectiveness evaluation Skill Matrix Authorization/ Appointment Letter Responsibilities List Job description List Qualifications List List of Competence requirements Equipment Procedure Traceability information (Metrological Traceability) Equipment Master list and their location having history and unique identificationnumber Details and calibration status of equipment form Preventive Maintenance Plan Corrective Action Calibration Schedule Statistical techniques Report Control chart Facilities and Environmental Conditions Monitoring Procedure Environmental conditions monitoring form Security Procedure Cleaning and safety procedure Housekeeping Checklist/record2. Process Requirements Externally provided products and services procedure 5 PageList of approved suppliers
Supplier Evaluation FormSupplier Re-Evaluation FormPurchase Request/Indent FormInspection form for Incoming Critical Supplies/EquipmentMaterial Acceptance/rejection form Review of requests, tenders and contracts Procedure Customer Service Request Form Handling, transport and storage procedure Equipment TagProcedure for receiving samplesProcedure for transportation of chemical/ consumable materialsProcedure for storage of sample/ chemical as well as for leftover/ remnant chemicalsAuthorization from state pollution control board for receiving, storage, transboundary movement and safe disposalProcedure for disposal and record of disposal of pesticides/chemicals Receiving and releasing procedure Incoming and Outgoing register Receiving Record Releasing Record Equipment Control Tag Certificate Receive form Technical records6 Page Accomplished Measurement Data Sheets Calibration Certificates Measurement Uncertainty calculation ( CRM & Equipment) Intermediate check records Preventive Maintenance Records Corrective Action record Validation Records Repeat analysis record
Records of Inter Laboratory Comparison Program Records of blind analysis Selection and Verification of Methods Procedure Verification and Validation Form Calibration Procedure List Technical worksheets (Measurement Data Record Sheet) Operating instructions/work instructions list International / National Standards List Validation of Methods Procedure Validation Check form Validation Report and Approval Form Evaluation of Measurement Uncertainty Procedure Measurement uncertainty budget and calculator Ensuring the validity of results7 Page Intermediate check procedure Intermediate check Form Preventive maintenance procedure Preventive maintenance plan Equipment History Card PT/ILC results PT/ILC Plan PT/ILC Analysis report Functionality check form Control Charts –see under Equipment Replicate Test Results Repeatability and Reproducibility Results Reporting of Results – requirements for certificates Calibration results (Measurement Data Sheet) Calibration certificate format which includes: Reporting statements of conformity guide Reporting opinions and interpretations guide
Amendments procedure Other Records and Forms which are used depending on the Laboratory Design Complaints ProcedureComplaints Record Form Procedure for handling of complaints Corrective and preventive action form Control of data and information management Procedure Customer feedback procedure, SOP and forms& format3. Management System Requirements Impartiality and confidentiality Procedure Confidentiality Acceptance formImpartiality policyRisk to impartiality monitoring sheetRisk Assessment records Management system documentation(a) Documents control procedure Change request form/Amendment formDocument for archive or disposal formMaster list and distribution list of documents(b) Records Control Procedure List of records Locations and retention period record(c) Internal Audit Procedure Internal Audit report Internal Audit non-conformity reportCorrective and preventive action formAudit plan Management Review Meeting Procedure8 Page
Management Review meeting form (agenda with attendance) Minutes of meeting Corrective Action Procedure Corrective action form Root cause analysis procedure, SOP and Forms & Formats Actions to Address risks and Opportunities Procedure Risk assessment database file Risk and Opportunity for Improvement Form Improvement Procedure Improvement monitoring sheet Control of Non-conforming Procedure Hold and release tag Service Report Form Customer Complaints Procedure Complaints and feedback form4. Prepare Quality Manual (QM) of the laboratory, as per the guidance provided in NABL documentNo-160, which gives an insight of the process for preparing Quality manual and Structure andformat of quality manual.5. Prepare quality system procedure (QSP) which define the management, quality system forms andformats (QSF&F), Standard Operation Procedure (SOP) and Work Instructions6. Prepare Standard Operating Procedure and Work Instructions for each and every test of pesticide,which a laboratory like to include in the scope of accreditation.7. Ensure effective environmental condition in the laboratory like temperature, humidity, storage andhouse keeping etc.8. Ensure the calibration of equipment and glassware from an accredited institute for calibration andmaintain their chain of traceability with preventive maintenance.9. Purchase certified reference material for the pesticides under scope of accreditation and maintaintheir traceability record.9 Page
10. Calculate the measurement of uncertainty for every procedure/equipment as per MU given bycertificate of calibration.11. Ensure proper implementation of all aspects that have been documented in the Quality Manual andother documents. The laboratory must ensure that the procedures described in the Quality Manualand other documents are being implemented and evaluated timely.12. The Laboratory must have participated satisfactorily in an inter-laboratory comparison withadequate number of accredited laboratories. The minimum stipulated participation for laboratoriesis one parameter/ type of test/ calibration per discipline, prior to grant of accreditation and an ongoing program as per NABL 163. The satisfactory performance shall be defined in term of z-scoreor any other acceptable internationally accepted method. For unsatisfactory performance, thelaboratory is to take corrective actions.13. Conduct at least one internal audit for Quality management and Technical Aspect in accordancewith ISO and Management review Meeting (MRM) followed by Management Review CommitteeMeeting (MRCM) of the laboratory before applying for accreditation.14. Laboratory has to maintain records of Internal audit as well as training documents of IA inrespective form and filesAccreditation ProcessAn applicant laboratory will apply on the website of NABL for submission of application and theconcerned fee. The Legal identity of laboratory, Authorised signatory and quality manual prepared bythe laboratory will be submitted online. For different discipline the laboratory has to apply separately.The Quality Manual will be forwarded by NABL to a Lead Assessor to judge the adequacy of theQuality Manual as to whether it is in compliance with ISO 17025 standards.Thereafter the Lead Assessor will conduct a Pre-Assessment of the laboratory for one day. Based on thePre-Assessment report the laboratory may have to take certain corrective actions, so as to be fullyprepared for the final assessment.Finally, when the laboratory is ready, the Lead Assessor and a team of technical assessors will conductthe final assessment. The number of technical assessors will depend on the number of disciplinesapplied for. The accreditation process involves a thorough assessment of all the elements of thelaboratory that contribute to the production of accurate and reliable test data. These elements includestaffing, training, supervision, quality control, equipment, recording and reporting of test results and theenvironment in which the laboratory operates. The laboratory may have to take certain correctiveactions, after the final assessment.After satisfactory corrective actions are taken by the laboratory (within a period of one month), theAccreditation Committee will examine the report and if satisfied recommend accreditation. The timerequired for the process of accreditation will depend upon the preparedness of the laboratory and itsresponse to the non - conformances raised during the pre-assessment and final assessment. The totalduration ranges between 6 and 8 months.10 P a g e
Surveillance and Re-AssessmentAccreditation to a laboratory shall be valid for a period of two years. The laboratories may enhance orreduce the scope of accreditation during surveillance. After every year, Lab has to apply for the desktopsurveillance audit which is conducted in NABL office itself for the work done by the Laboratory tocheck the technical competence to comply ISO 17025 standard.The laboratories need to apply for renewal of accreditation, at least six months before the expiry ofvalidity of accreditation for which a re-assessment shall be conducted.The information on fees of accreditation and its further maintenance may be gathered from NABLdocument No 100.References:1.ISO/IEC 17025:2017 , General requirements for the competence of testing and calibrationlaboratories. International Organization for Standardization by ISO Central Secretariat Ch. deBlandonnet, 8 Case Postale, 401 CH – 1214 Vernier, Geneva, Switzerland2.Laboratory accreditation-Procedural guidelines, A.S .Kanagasabapathy and Pragna RaoKamineni Hospitals Ltd., L.B. Nagar, Hyderabad - 500 068, Indian Journal of ClinicalBiochemistry,2005, 20 (2) 186-1883.www.nabl-india.org11 P a g e
Important documents related to accreditation available on the website of NABLS. No.Name of the documentDocument NoGeneral Information BrochureTerms & Conditions for Obtaining andMaintaining NABL AccreditationApplication Form for Testing LaboratoriesNABL’s Policies for Accreditation(as per ISO/IEC 17025:2017Guide for Preparing Quality ManualGuidelines for Estimation and Expression ofUncertainty of Measurement1001317.Policy on Calibration and Traceability of Measurements1428.Policies and Procedures for inter-laboratorycomparisons and / or Proficiency Testing1639.Pre-assessment Guidelines and Forms20910.Policy and Procedures for assessment,Surveillance & Re-assessment of laboratoriesPolicies & Procedures for dealing withAdverse Decisions214Master list of NABL documents NABLDocument Review Checklist (as per ISO/IEC 17025:2017)Desktop surveillance2002201.2.3.4.5.6.11.12.13.14.12 P a g e151165160141216218
The laboratory accreditation services to testing and calibration laboratories are provided by NABL in accordance with ISO 17025: 2017 'General Requirements for the Competence of Testing and Calibration Laboratories Requirements and procedure for getting accreditation from NABL Getting ready for accreditation