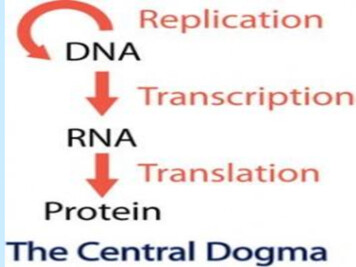
Transcription
DNA Replication and Repair – Sep. 26-28, 2007Dr. Jim Borowiec (MSB383A)(E-mail: James.Borowiec@med.nyu.edu)I. DNA ReplicationA. Requirement for high-fidelity DNA replicationB. Enzymology and mechanism of DNA synthesis1. General description of replication process2. Properties of DNA polymerases3. Therapeutic nucleotides4. Okazaki fragment synthesisPriming and processing5. Other replication factorsSliding clamps, DNA helicases, etc.C. Initiation of DNA replicationD. TelomeresII. DNA RepairA. Types of DNA damageSpontaneous and environmentalB. Base excision repairDNA glycosylases, AP endonucleaseC. Nucleotide excision repairE. coli UvrABC endonuclease, helicase II (uvrD)Human xeroderma pigmentosum (XP) complementation groupsD. Mismatch repairE. coli mutS, mutH, mutL and determination of incorrect strandhuman hMSH2, hMLH1 and hereditary nonpolyposis colon cancerE. Defects in cell cycle checkpointsAtaxia Telangiestasiap53 mutation1
Overview of DNA replicationTelomereCentromereTelomereDNA chromosomeOrigin ofDNA replicationSpecialized elements termed'origins of DNA replication'occur many times on achromosomeInitiation of DNA replicationfrom origin of replicationgenerates structures termed'DNA replication bubbles'.(One of many replicationbubbles is shown)direction offork movementNascent(daughter) strandsParental strandsEach replication bubblecontains two 'DNAreplication forks' movingin opposite directions.(An enlarged view of one forkis shown here)DNA replicationcomplex (bound to fork)Action of DNA replicationcomplex (acting on fork)duplicates both parental strands 2
Continual Priming of Okazaki Fragments3'Nascentleading DNA5'RNA primerNascentlagging DNALEADING STRAND5'3'3'FORK MOVEMENT3'5'LAGGING STRANDPriming5'3'New priming eventExtension5'3'Primer extensionProcessing5'3'Removal of RNA primerand ligationReplication employs two different mechanisms to replicate the leading and laggingstrands. DNA synthesis on the leading strand occurs in the same direction as fork movement, iscontinuous and generally highly processive. Lagging strand DNA synthesis occurs in thedirection opposite to fork movement, is discontinuous and uses DNA polymerases with moderateprocessivity.3
The DNA polymerase enzyme. The structure of various DNA polymerases have beensolved by X-ray crystallography. These structures indicate that the ‘typical’ DNApolymerase resembles a human right hand, in which the thumb, palm, and fingers claspthe DNA. In this way, the DNA is properly positioned for association with thepolymerase active site and catalytic efficiency is deoxyribonucleosideP 3'triphosphateP OH3'P P POHP P P P P P3'5'P5'’2 PiP3'P P P POH5'-to-3'direction ofchain growthP P P P P P3'5'Nascent strand synthesis by DNA polymerases occurs only in the 5’ to 3’direction. Nucleotide incorporation utilizes two high-energy phosphate bonds, drivingthe reaction in the forward direction.4
HHNORMAL C:G base pairNHNNHOOBASE(favored)NHCytosine(amino form)NNNH NNONH NHBASE*(rare)ABNORMAL C*:A base pairHHNNHHNONHCytosine*(imino form)NNHNNHNNOHN Anomalous base pairing can occurthat involves rare tautomeric forms of thebases. In the example shown, when cytosineis in the rare imino form (C*), it can form abase pair with the common amino form ofadenine (A). The rare form of adenine (A*)can also pair with the common form ofcytosine (C). G*:T and G:T* base pairs canalso form. Defective base insertion can becorrected either by DNA polymeraseproofreading or mismatch repair.An important process required for high fidelity DNAreplication is proofreading. DNA molecules with amismatched nucleotide at the 3′ end of the primerstrand (i.e., because of DNA polymerasemisincorporation) are poor templates for continuedDNA synthesis and usually cause stalling of the DNApolymerase.A 3′-to-5′ proofreading exonucleaseassociated with the polymerase enzyme removesunpaired nucleotides from the 3′ end of the nascentstrand. The DNA polymerase can therefore act as aself-correcting enzyme to remove its ownpolymerization mistakes.5
The activities of key replication enzymes are conserved inbacteria (E. coli) and eukaryotes (human). For example, theoverall structure of the E. coli β protein and the human PCNA aresimilar, even though the gene sequences are divergent.6
Polymerase nameFunctionDNA polymerase α(pol α)Primer function in DNAreplicationBase excision repairpol βMitochondrial DNAreplication and repairDNA replication; nucleotideand base excision repairDNA replication; nucleotideand base excision repairTranslesion synthesis?pol γpol δpol εpol ζAccurate translesionsynthesisDNA repair of crosslinks?pol ηpol θSomatic hypermutationpol ιError-prone repairpol κMeiosis-associated DNArepair?Somatic hypermutation?pol λpol µHuman DNA-directed DNA polymerases. Human cells, similar to prokaryotic and othereukaryotic cells, contain multiple DNA polymerases. The activities of these DNApolymerases differ to a certain extent because of the specific roles they play in DNAreplication, repair, and recombination. The most important human DNA polymerases forgeneral DNA replication are DNA polymerase α (which has an associated DNA primaseactivity) and DNA polymerase δ. DNA polymerase δ is the major DNA polymerase requiredfor processive leading strand synthesis, but also functions to complete Okazaki strandsynthesis on the lagging strand template. DNA polymerases α to ε have been known for anumber of years and are fairly well characterized. Most of the other DNA polymerases havebeen identified only very recently, and the characterization of their functional role isongoing. Note that the list is not comprehensive – additional DNA polymerases have beenidentified which are not included in this list.7
Therapeutic nucleoside analogs1. AnticancerHHCytosineOH O- CH 2OHHConverted to the nucleoside triphosphate. Inhibitselongation of DNA synthesis by competing with dCTPfor DNA polymerase. Can act as a chain terminator selectively kills growing cells. Antileukemic.OH Hcytosine arabinoside (araC)2. AntiviralA. Anti-HIVH O- CH 2HThymineOHHHConverted to the nucleoside triphosphate. Selectivelyinhibits HIV reverse transcriptase activity (an essentialHIV DNA polymerase that synthesizes the HIV DNAstrands from the RNA template). Acts as a chainterminator.N3 H3'-deoxy-3'-azidothymidine (AZT)InosineH O- CH 2 OHHHHHHdideoxyinosine (ddI)Inosine is a precursor to purines guanine and adenine.Converted to the nucleoside triphosphate. Selectivelyinhibits HIV reverse transcriptase activity. Acts as a chainterminator.B. Other Anti-ViralH O- CH 2HOGuanineH H HAcyclovirPotent inhibitor of herpes simplex viruses (HSV).Converted to the triphosphate. Inhibits activity of HSVDNA polymerase while having lesser effect on cellularDNA polymerases. Acts as a chain terminator.8
Okazaki fragment (OF) synthesis in human cellsNorma lly occurs on lagging strandTwo DNA polymeras es are usedRNA primer synthesisby DNA primase (assoc.with D NA pol ")3'Step 1. Priming5' end of pr evious OFFORK MO VEMENTiDNA synthesisby pol "5'LAG GIN G STRANDTEMP LATEDNA pol "Step 2. Synthes is of initiatorDNA (iDNA).3'5'DNA pol !Synthe sis of body of OFby pol !DN A polym erases can not star tna scent strand synthesis de novo.Need RNA priming by DNA primaseassoc iated with D NA pol ". Primeris 10 nt in length.Nick in D NA(contains 3'-O H)3'5'RNa se H nucl ease3'DN A pol " extends the RNAprimer. T he iD NA is 35 nt inlength, and acts as aDN A pr imer.Step 3. Extension of iDNA.DN A pol ! (which has nopriming activity) extends the iD NA.Extension continues 200 nt to 5'en d of previous OF.Step 4. Removal of RNA prim er.5'The RNA primer is remove d byRNase H (which cleaves the RNAportion of a DNA- RNA hy brid).Most of the iDNA is also removedby a nuclea se. Occurs while nextOF is being syn the sized.DNAligaseStep 5. Ligation.3'5'9In the final reaction, the OFsare joine d
A HumanB E. coliRNAprimerRPA1previous Okazakifragment3'5' Lagging strand template3'1RNAprimerSSB3'5' Lagging strand template3'pol α /primaseprevious OkazakifragmentprimasePriming and DNA3' synthesis by5' pol α /primase25'3'3' Recognition by PCNA5' and DNA pol δ35'3'3' Recognition by pol III5' holoenzyme3'5' Elongation45'3'3' Elongation5'25'3'35'3'45'3'55'3'3' Removal of RNA primer5' by RNase H/nuclease55'3'3' Removal of RNA primer5' by RNase H/pol I65'3'3' Gap-filling5'65'3'3' Gap-filling5'75'3'3' Joining of Okazaki5' fragments75'3'PCNA /pol δ3' RNA primer synthesis by5' primasepol III holoenzymePCNA/pol δRNase HRNase HDNA ligaseDNA ligase3' Joining of Okazaki5' fragmentsEukaryotic and prokaryotic replication utilize a generally similar mechanism of laggingstrand synthesis. In the case of the eukaryotic replication, the DNA polymerase alpha/DNAprimase (pol α/primase) first makes a short RNA/DNA hybrid, which DNA polymerase delta(pol δ) then extends. Processing enzymes including RNase H and DNA ligase join the Okazakifragments. For prokaryotic DNA synthesis, primase synthesizes the RNA primer, which then isused by the pol III holoenzyme complex. A second polymerase (pol I), in combination withRNase H, processes the 5’ end of the previous Okazaki fragment. After the pol III holoenzymefills the gap, DNA ligase joins the two strands. In each case, therefore, two distinct DNApolymerases are used for lagging strand synthesis.The proteins at a DNA replication fork. The major types of proteins thatact at a DNA replication fork are illustrated, showing their positions on the DNA.10
Three-dimensional view of a replication fork. Current work indicates that, both forprokaryotic and eukaryotic DNA replication, the primary leading strand and lagging strandDNA polymerase are coupled. For human DNA replication (shown), two molecules of DNApolymerase delta (pol δ) are joined. Upon completion of the Okazaki fragment, the laggingstrand pol δ would transfer to the most recently formed RNA/DNA primer (synthesized by theDNA polymerase α/DNA primase complex; pol α/primase). For the E. coli system, twomolecules of the pol III holoenzyme would be joined. Because the lagging strand loop wouldvary in size during the synthesis of each Okazaki fragment, this mode of DNA synthesis hasbeen termed the ‘trombone model’ of DNA synthesis.MitosisGap 2MG2G1Gap 1SDNA synthesisThe eukaryotic cell cycle generally has four phases termed M (mitosis andcytokinesis), G1 (first gap phase), S (DNA synthesis), and G2 (the second gap phase).Chromosomal DNA replication occurs during S phase. During S phase, all genes arereplicated only a single time (the ‘once and only once’ mechanism of eukaryotic DNAreplication). Because of this type of regulation, no genes are lost (because they were notreplicated) or re-duplicated (because they underwent a second round of replication during asingle S phase).11
Mechanisms of topoisomerase I and topoisomerase II activity. In each case, the topoisomerasemakes reversible covalent bonds with the DNA. Each topoisomerase activity has somewhatdifferent roles. Topoisomerase I serves to relieve the torsional strain ahead of the replicationfork generated as a result of DNA unwinding. Topoisomerase II generally separates the twodaughter molecules at the final stages of DNA replication (and just prior to mitosis). Because oftheir critical roles during DNA replication, various therapeutic agents are directed against boththese enzymes.12
Mechanism for replication initiation at oriC, the replication origin for E. coli. The dnaA proteinfirst binds specific sequences in oriC (the 9-mers). In the presence of ATP, dnaA causes alocalized melting of DNA elements that have a relatively unstable duplex (the 13-mers) to formthe open complex. The replicative DNA helicase (dnaB) can then joins the complex and extendsthe melted bubble. The remainder of the DNA polymerase machinery can then bind to theexposed single-strand and catalyze nascent strand synthesis.Mechanism for replication initiation in eukaryotic cells. The reaction mechanism is significantlymore complex than used for prokaryotic initiation. The origin recognition complex (ORC) firstrecruits cdc6 and the MCM complex to the origin during the G1 phase. At the G1/S phasetransition, cell cycle kinases (e.g., cdk2/cyclin E and cdc7/dbf4) become activated andphosphorylate the origin-bound proteins. Other replication factors are subsequently recruitedleading to melting of the origin DNA and loading of the DNA polymerase machinery. Many ofthe details of the process are still not understood.13
Replication fork initiation. The replicationinitiation complex binds origin sequences andinduces the local opening of the DNA duplex. Inthe case of eukaryotic DNA replication, the DNApol α/primase complex then binds and synthesizesan RNA/DNA primer to initiate leading strandsynthesis. The formation of this replication forkgenerates longer ssDNA regions that serve to bindadditional replication components. In this way, tworeplication forks are formed, moving in oppositedirections, that synthesize both nascent leading andlagging strands.Telomeres and telomerase. The ends of linear chromosomes in eukaryotes are ‘capped’by repeating G-rich sequences, which comprise telomeres. For certain ciliates (shown), therepeats have a GGGTTG sequence, but in humans they are GGGTTA. The shorter bottomstrand was initially synthesized as a nascent lagging strand, but is shortened upon processing(i.e., by removal of the RNA/iDNA primer). Without telomerase, therefore, each chromosomeloses a few telomere repeats and becomes shorter every cell cycle. Telomerase contains anRNA template which allows the synthesis of more telomere repeats at the 3’ end of telomericDNA. Thus, shortening (which could eventually lead to loss of genetic information) isprevented.14
Germ line cellsSomatic cellsp53 mutationTelomerase activationHayflick limitTelomere stabilizationWidespread cell deathSenescence Crisis# of cell divisionsTelomere shortening in the absence of telomerase. Normal human somatic cells expressvery low levels of telomerase. As a person ages, the telomeres shorten because post-replicationprocessing (particularly the removal of the RNA primer from the lagging strand product)results in the loss of DNA. Over many generations, the telomere length becomes sufficientlyshort (the Hayflick limit) that it causes cells to become senescent, and cells will no longerdivide. This may be an evolutionary device to inhibit cell immortalization and tumorigenesis. Ifcells lack p53 (p53 is often mutated in cancer cells), they do not undergo senescence butinstead continue to divide. In these cells, telomere length becomes so short that it causes cellsto undergo apoptosis (crisis phase). Occasionally, a rare cell survives which expressestelomerase activity and can therefore become immortal. In contrast to somatic cells, germlinecells and almost all tumor cells express telomerase and maintain telomere length. Becausetelomerase activation appears to be a critical step in cell immortalization, anti-telomeraseagents are being developed that could potentially lead to novel treatments against cancer cells.15
Overview of DNA RepairNH 2HNOONH 4 HNHHNOH CytidineH 2OUridineUracil-DNA glycosylaseOHO sugar-phosphatebackboneOAP siteHHNNHHFree uracilBase damage. A very common type of DNA damage is spontaneous deamination. Inthe above example, the cytidine to uridine conversion occurs at a rate of 100 basesper genome per day. Other common spontaneous types of DNA damage aredepurination and depyrimidation (creating AP sites), and oxidative damage to bases.Base damage also occurs from environmental sources such as ionizing or ultravioletradiation, and chemical agents. If the damage occurs on a single base and does notresult in a bulky adduct, it is repaired by base excision repair (BER). In the first stepof BER, a DNA glycosylase (many different DNA glycosylases exist) hydrolyzes theN-glycosidic bond between the damaged base and the sugar-phosphate backbone,giving an AP site and releasing the free base.16
Schematic representation of base excision repair. For simplicity, only the relevant DNAstrand is shown for most of the figure. Certain forms of base damage are recognized by DNAglycosylases (step 1), that catalyze excision of the free base by hydrolysis of the N-glycosylbond linking the base to the sugar-phosphate backbone. This reaction leaves anapurinic/apyrimidinic (AP) site in the DNA. Attack at such sites by an AP endonuclease (step2) results in a strand break with a 5’ phosphate and a 3’ hydroxyl. The abasic sugarphosphate residue is excised by a phosphodiesterase. The resulting single nucleotide gap isfilled by repair synthesis, and DNA repair is completed by DNA ligase.The exposure of cells to UV light (such as contained in sunlight) can result in theformation of thymine dimers in chromosomal DNA. The formation of these dimers isexcised in the process of nucleotide excision repair, involving the UvrABC endonucleasein E. coli (below), and XP proteins in humans.17
Nucleotide excision repair in bacteria. Thisrepair process excises thymine dimers (shown) fromDNA although other bulky adducts are alsosubstrates for this reaction. In the first step (A), thethymine dimer (T T) is recognized by the UvrAand UvrB proteins. UvrC replaces UvrA (step B)and the UvrB/UvrC complex nicks the DNA strandon either side of the damage (step C). In the nextstep D, Helicase II (also called uvrD) removes theexcised strand (12 to 13 nts in length). The gap isthen filled by DNA polymerase I (pol I) and thestrands joined by the action of DNA ligase. Therecognition/excision proteins are usually termed theUvrABC endonuclease. Model for the strand-specifictranscription-coupled DNA repair in E. coli.(A) RNA polymerase is shown transcribing atemplate strand that contains base damageahead of the transcription complex. (B to D)Stalling of RNA polymerase at the site of basedamage in the transcribed strand (B) results inthe binding of the transcription-repair couplingfactor (TRCF; C) and displacement of thepolymerase and the truncated transcript,leaving TRCF bound at the site of damage (D).TRCF then binds the UvrABC endonuclease(E) and nucleotide excision repair takes place(F).18
Nucleotide Excision RepairRole in RepairFactorXPADamage recognitionRPADamage recognitionTFIIH (XPB, XPD)Formation of preincision complexXPCStabilization of preincision complexXPF5’ excisionXPG3’ excisionProteins required in human nucleotide excision repair (NER). Most repairfactors were named after xeroderma pigmentosum (XP), an inherited disease inwhich patients show an extreme sensitivity to sunlight. The process of NER inhumans is generally similar to NER in bacteria although the excised fragment islarger (the predominant species 27 to 29 nts is in length).Microsatellite Instability. Normal human cells show a low level ofpolymorphism of repeat or ‘microsatellite’ sequences such as (CA)n. Occasionalslippage is corrected by mismatch repair. Cells from hereditary nonpolyposis coloncancer (HNPCC) patients show a greatly increased level of sequence instability,often resulting from mutation of mismatch repair genes (usually hMSH2 andhMLH1). In the figure, a specific DNA region containing a polymorphic site wasamplified by PCR and the products were separated by gel electrophoresis. The PCRproducts of colon cancer tissue DNA (T) from seven different HNPCC patients orfrom adjacent normal tissue DNA (N) are shown. For most of the patients, the tumortissue shows an increased level of polymorphism at these (and other) sites, comparedto normal tissue.19
Mismatch repair in bacteria. Three E. coli proteins (mutS, mutL, andmutH) are primarily responsible for mismatch recognition. The mutH proteinnicks the DNA strand containing the incorrect base at a distal site, and that strandis then degraded back to the mismatch. Damage to the genes coding for theirhuman counterparts appears to trigger hereditary colon cancer and other forms ofcancer.20
21
Dr. Jim Borowiec (MSB383A) (E-mail: James.Borowiec@med.nyu.edu) I. DNA Replication A. Requirement for high-fidelity DNA replication B. Enzymology and mechanism of DNA synthesis 1. General description of replication process 2. Properties of DNA polymerases 3. Therapeutic nucleotides 4. Okazaki fragment synthesis Priming and processing 5.