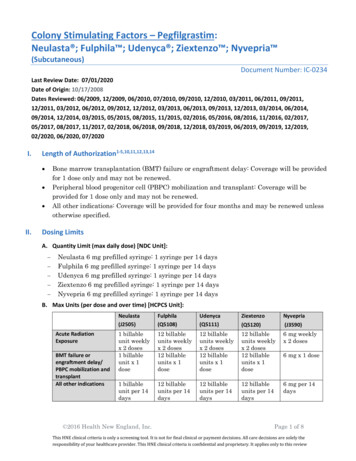
Transcription
NYVEPRIA (pegfilgrastim-apgf)Billing and Coding GuidePlease see Important Safety Information and Indication on pages 11-12 and full Prescribing Information, Patient Information, and Instructions for Use forNYVEPRIA at NyvepriaHCP.com.Please see Important Safety Information and Indication on pages 11-12 and full Prescribing Information, Patient Information, and Instructions for Use forNYVEPRIA at NyvepriaHCP.com.NYVEPRIA is a trademark of Pfizer Inc.Pfizer Oncology Together is a trademark of Pfizer Inc.
IntroductionPfizer Inc. has developed this reference guide to assist healthcare providers (HCPs) with understanding coding for NYVEPRIA (pegfilgrastim-apgf), apegfilgrastim biosimilar approved for use in the United States for subcutaneous injection.The information provided in this document is intended for informational purposes only and is not a comprehensive description of potentialcoding requirements for NYVEPRIA. Coding and coverage policies change periodically and often without notice. The HCP is solely responsible fordetermining coverage and reimbursement parameters and appropriate coding for treatment of his/her patients. The information provided shouldnot be considered a guarantee of coverage or reimbursement for NYVEPRIA.Please see Important Safety Information and Indication on pages 11-12 and full Prescribing Information, Patient Information, and Instructions for Use forNYVEPRIA at NyvepriaHCP.com.2
Making your patients’ support needs a priority. Together.At Pfizer Oncology Together , patient support is at the core of everything we do. We’ve gathered resources and developed tools to help patients and their lovedones throughout NYVEPRIA treatment. From helping to identify financial assistance options to connecting patients to resources for emotional support, yourpatients’ needs are our priority.*Benefits VerificationWe can help determine a patient’s coverage and out-of-pocket costs.Prior Authorization (PA) AssistanceWe can coordinate with a patient’s insurer to determine the PA requirements. After a PA request issubmitted, we can follow up with the payer until a final outcome is determined.Appeals AssistanceWe can review the reasons for a denied claim and provide information on payer requirements. After anappeal is submitted, we can follow up with the payer until a final outcome is determined.Billing and Coding Assistance for Injectable ProductsFor your patient claim submissions, we provide easy access to sample forms and template letters, alongwith billing and coding information for physician office and hospital outpatient settings of care.Patient Financial AssistanceWe can help patients understand their benefits and connect them with financial assistance resources.FOR LIVE, PERSONALIZED SUPPORTCall 1-877-744-5675 (Monday–Friday 8 AM–8 PM ET)VISITPfizerOncologyTogether.com*Some services are provided through third-party organizations that operate independently and are not controlled by Pfizer. Availability of services and eligibility requirements are determinedsolely by these organizations.Please see Important Safety Information and Indication on pages 11-12 and full Prescribing Information, Patient Information, and Instructions for Use forNYVEPRIA at NyvepriaHCP.com.3
Coding OverviewIt is critical to report billing codes that accurately reflect a patient’s condition, treatment, and the services that are rendered on the claim form submittedto a payer. The codes in this section may be appropriate to report services related to therapy with NYVEPRIA (pegfilgrastim-apgf) when performed in thephysician office and hospital outpatient department sites of care to Medicare Administrative Contractors (MACs), private commercial payers, and Medicaid.Coding for NYVEPRIAIn the physician office and hospital outpatient department sites of care, Medicare, Medicaid, and private commercial payers typically recognize the followingcodes for reporting NYVEPRIA and its administration on claim forms.Effective for dates of service on and after January 1, 2021, HCPCS code Q5122 may be used to report NYVEPRIA.HCPCS Code1Q5122DescriptorInjection, pegfilgrastim-apgf, biosimilar, (nyvepria), 0.5 mgModifiers may be included on claims to provide additional information. Some payers may require the modifier JB to be reported, indicating a subcutaneousroute of administration. Additional modifiers may also be considered appropriate when submitting claims.HCPCS Modifier2JB JADescriptorSubcutaneous administrationPlease see Important Safety Information and Indication on pages 11-12 and full Prescribing Information, Patient Information, and Instructions for Use forNYVEPRIA at NyvepriaHCP.com.4
NYVEPRIA National Drug CodeNational Drug Codes (NDCs) are unique 10-digit, 3-segment numbers used to identify drugs.3 State Medicaid agencies usually require 11-digit NDCs onclaims, even after a unique code has been assigned.Strength4Prefilled Syringe Size10-Digit NDC6 mg/0.6 mLSingle-dose prefilled syringe for manual use only0069-0324-01NDC Conversion ExampleFor reimbursement purposes, some payers (eg, Medicaid) require the NDC on the claim form. For claims-reporting purposes, the Health Insurance Portabilityand Accountability Act (HIPAA) requires conversion of the 10-digit NDC to an 11-digit NDC by adding a leading “0” (zero), where appropriate, to create a 5-4-2configuration. The zero is added in front of the first segment of numbers when the 10-digit format is the 4-4-2 configuration for NYVEPRIA. See placement ofthe red zero in the example below.StrengthPrefilled Syringe Size10-Digit NDC11-Digit NDC6 mg/0.6 mLSingle-dose prefilled syringe formanual use only0069-0324-0100069-0324-01Please see Important Safety Information and Indication on pages 11-12 and full Prescribing Information, Patient Information, and Instructions for Use forNYVEPRIA at NyvepriaHCP.com.5
Coding for NYVEPRIA Administration ServicesCurrent Procedural Terminology (CPT ) codes define specific medical procedures performed by physicians or other qualified HCPs.5 The following codes may beused to report the subcutaneous administration of NYVEPRIA:Type of CodeCPT code5Code/Descriptor96372: Therapeutic, prophylactic, or diagnostic injection (specify substance or drug);subcutaneous or intramuscularRelevant Sites of ServicePhysician office and hospitaloutpatient departmentHospital outpatient departments use revenue codes to report specific accommodations and/or ancillary charges.6Type of CodeCode/DescriptorRelevant Site of Service0636: Drugs requiring specific identification – detailed codingUsed in combination with the drug codeRevenue code70500: Outpatient services – general classificationUsed in combination with the administration codeHospital outpatient department0510: Clinic – general classificationUsed in combination with the administration codeCurrent Procedural Terminology (CPT ) is a registered trademark of the American Medical Association.Please see Important Safety Information and Indication on pages 11-12 and full Prescribing Information, Patient Information, and Instructions for Use forNYVEPRIA at NyvepriaHCP.com.6
Diagnosis Coding for NYVEPRIAThe International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code set should be used, as appropriate, to report thepatient-specific diagnosis documented in the medical record.Reporting the medical necessity for NYVEPRIA may require a primary and secondary diagnosis, in some cases. HCPs should verify payer-specific diagnosiscoding and sequencing requirements before submitting a claim, as they may vary by payer.NYVEPRIA (pegfilgrastim-apgf) is a biosimilar that is FDA-approved to decrease the incidence of infection, as manifested by febrile neutropenia, in patientswith non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia.Limitations of UseNYVEPRIA is not indicated for the mobilization of peripheral blood progenitor cells for hematopoietic stem cell transplantation.Consult your reimbursement expert for appropriate codes.Please see Important Safety Information and Indication on pages 11-12 and full Prescribing Information, Patient Information, and Instructions for Use forNYVEPRIA at NyvepriaHCP.com.7
Sample Claim Form: CMS-1500, Physician Office Site of ServiceELPMASItem 21: Specify appropriateICD-10-CM diagnosis code(s)This sample form is intended as a reference for the coding andbilling of NYVEPRIA. This form is not intended to be directive,and the use of the recommended codes does not guaranteereimbursement. HCPs may deem other codes or policies moreappropriate and should select the coding options that mostaccurately reflect their internal guidelines, payer requirements,practice patients, and services rendered.Item 19: Enter the appropriatedrug-identifying information as required bypayer (eg, brand/generic drug name, NDC11-digit format, dose administered, route ofadministration, etc)XXX.XXXXMM DDYYMMDDYY1122A12MM DDYYMMDDYY112A1Item 24D: Specify appropriate HCPCSand CPT codes and modifiers, forexample: Drug: Q5122 for NYVEPRIA Administration: 96372 foradministrationPLEASE PRINT OR TYPEItem 24G: Specify the billing units. Forexample, Q5122 billing unit 0.5 mg ofpegfilgrastim-apgf biosimilar (NYVEPRIA).To report 6 mg, bill Q5122 with 12 units.To bill for the subcutaneous injection, bill1 unit of 96372.Item 24E: Enter reference to thediagnosis for the CPT and HCPCS codesfrom Item 21APPROVED OMB-0938-1197 FORM 1500 (02-12)Please see Important Safety Information and Indication on pages 11-12 and full Prescribing Information, Patient Information, and Instructions for Use forNYVEPRIA at NyvepriaHCP.com.8
Sample Claim Form: UB-04, Hospital Outpatient Site of ServiceThis sample form is intended as a reference for the coding andbilling of NYVEPRIA. This form is not intended to be directive,and the use of the recommended codes does not guaranteereimbursement. HCPs may deem other codes or policies moreappropriate and should select the coding options that mostaccurately reflect their internal guidelines, payer requirements,practice patients, and services rendered.Form Locator (FL) 44: Specify appropriate HCPCSand CPT codes and modifiers, for example: Drug: Q5122 for NYVEPRIA Administration: 96372 for drug administration0636000636: Drugs requiring specific identification –detailed coding ( or)linic – general classification ( orin ection ad inisteredin t e clinic)22MM DD YY96372MM DD YY2ELPSAMFL 46: Specify the billing units. Forexample, Q5122 billing unit 0.5 mgof pegfilgrastim-apgf biosimilar(NYVEPRIA). To report 6 mg, billQ5122 with 12 units. To bill for thesubcutaneous injection, bill 1 unitof 96372.FL 42 and 43: Specify revenue codes and describe procedures, for example: 0636: Drugs requiring specific identification – detailed coding (for NYVEPRIA) 0500: Outpatient services – general classification 0510: Clinic – general classification (for SC injection administered in the clinic)Note: Other revenue codes may apply.FL 67: Specify appropriateICD-10-CM diagnosis code(s)xxx.xxxxFL 80: Enter the appropriate drug-identifyinginformation as required by payer (eg, brand/genericdrug name, NDC 11-digit format, dose administered,route of administration, etc)Please see Important Safety Information and Indication on pages 11-12 and full Prescribing Information, Patient Information, and Instructions for Use forNYVEPRIA at NyvepriaHCP.com.9
Claims Submission ChecklistThe following may be considered to assist with submitting claims completely and accurately, which is important for timely claims processing, appropriatepayment, and to avoid denied claims.Provide the patient name, address, and insuranceidentification number, and review each of these for accuracyInclude the HCP’s name, National Provider Identifier (NPI),and payer-specific provider ID (if applicable)Indicate the appropriate place of service code (2-digit code)for where the treatment was providedCheck to ensure that ICD-10-CM diagnosis codes, CPTprocedure codes, and modifiers (if applicable) are consistentwith information included in the patient’s medical recordReview the NYVEPRIA-specific information (eg, name of drug,HCPCS code, NDC, number of units, route, and frequency ofadministration)References1.Centers for Medicare & Medicaid Services (CMS). CMS HCPCS Application Summaries and Coding Decisions: Third Quarter, 2020 Coding Cycle for Drug and Biological Products. pdf. Accessed November 11, 2020.2.American Academy of Professional Coders (AAPC). 2020 HCPCS Level II Expert. Salt City, Utah: AAPC; 2019.3.U.S. Food and Drug Administration (FDA). National Drug Code directory. 2438.htm. Accessed February 17, 2020.4.NYVEPRIA [package insert]. New York, NY: Pfizer Inc.; 2020.5.American Medical Association. 2020 CPT Professional Edition. Current Procedural Terminology (CPT ) copyright 2019 by the American Medical Association. All rights reserved. Chicago, IL: AMA; 2019.6.Centers for Medicare & Medicaid Services (CMS). Transmittal 167. April 30, 2004. nce/Transmittals/downloads/R167CP.pdf. Accessed February 17, 2020.7.Research Data Assistance Center (ResDAC). Revenue center code. February 2008. Revenue%20Center%20Code%20Table.txt. Accessed February 17, 2020.Please see Important Safety Information and Indication on pages 11-12 and full Prescribing Information, Patient Information, and Instructions for Use forNYVEPRIA at NyvepriaHCP.com.10
IMPORTANT SAFETY INFORMATIONCONTRAINDICATIONS NYVEPRIA is contraindicated in patients with a history of serious allergicreactions to pegfilgrastim products or filgrastim products Reactions have included anaphylaxisWARNINGS AND PRECAUTIONSSPLENIC RUPTURE Splenic rupture, including fatal cases, can occur following theadministration of pegfilgrastim products Evaluate for an enlarged spleen or splenic rupture in patients who reportleft upper abdominal or shoulder pain after receiving NYVEPRIAACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) ARDS can occur in patients receiving pegfilgrastim products Evaluate patients who develop fever and lung infiltrates or respiratorydistress after receiving NYVEPRIA Discontinue NYVEPRIA in patients with ARDSSERIOUS ALLERGIC REACTIONS Serious allergic reactions, including anaphylaxis, can occur in patientsreceiving pegfilgrastim products The majority of reported events occurred upon initial exposure Allergic reactions, including anaphylaxis, can recur within days after thediscontinuation of initial anti-allergic treatment Permanently discontinue NYVEPRIA in patients with serious allergic reactions Do not administer NYVEPRIA to patients with a history of serious allergicreactions to pegfilgrastim products or filgrastim productsUSE IN PATIENTS WITH SICKLE CELL DISORDERS Severe and sometimes fatal sickle cell crises can occur in patients withsickle cell disorders receiving pegfilgrastim products Discontinue NYVEPRIA if sickle cell crisis occursGLOMERULONEPHRITIS Glomerulonephritis has occurred in patients receiving pegfilgrastim products The diagnoses were based on azotemia, hematuria (microscopic andmacroscopic), proteinuria, and renal biopsy Generally, events of glomerulonephritis resolved after dose-reduction ordiscontinuation of pegfilgrastim products If glomerulonephritis is suspected, evaluate for cause. If causality is likely,consider dose-reduction or interruption of NYVEPRIALEUKOCYTOSIS White blood cell counts of 100 x 109/L or greater have been observed inpatients receiving pegfilgrastim products Monitoring of complete blood count (CBC) during NYVEPRIA therapy isrecommendedCAPILLARY LEAK SYNDROME (CLS) CLS has been reported after granulocyte-colony stimulating factor (G-CSF)administration, including pegfilgrastim products, and is characterized byhypotension, hypoalbuminemia, edema, and hemoconcentration Episodes vary in frequency and severity and may be life-threatening iftreatment is delayed Patients who develop symptoms of CLS should be closely monitored andreceive standard symptomatic treatment, which may include a need forintensive carePOTENTIAL FOR TUMOR GROWTH STIMULATORY EFFECTS ONMALIGNANT CELLS The G-CSF receptor through which pegfilgrastim and filgrastim productsact has been found on tumor cell lines The possibility that pegfilgrastim products act as a growth factor for anytumor type, including myeloid malignancies and myelodysplasia, diseasesfor which pegfilgrastim products are not approved, cannot be excludedContinued on the next pagePlease see full Prescribing Information, Patient Information, and Instructions for Use for NYVEPRIA at NyvepriaHCP.com.11
IMPORTANT SAFETY INFORMATION (Continued)AORTITIS Aortitis has been reported in patients receiving pegfilgrastim products. Itmay occur as early as the first week after start of therapy Manifestations may include generalized signs and symptoms such asfever, abdominal pain, malaise, back pain, and increased inflammatorymarkers (e.g., c-reactive protein and white blood cell count) Consider aortitis in patients who develop these signs and symptomswithout known etiology. Discontinue NYVEPRIA if aortitis is suspectedNUCLEAR IMAGING Increased hematopoietic activity of the bone marrow in response togrowth factor therapy has been associated with transient positive boneimaging changes. This should be considered when interpreting boneimaging resultsMOST COMMON ADVERSE REACTIONS Bone pain Pain in extremityINDICATIONNYVEPRIA is indicated to decrease the incidence of infection, as manifestedby febrile neutropenia, in patients with non-myeloid malignancies receivingmyelosuppressive anti-cancer drugs associated with a clinically significantincidence of febrile neutropenia.Limitations of UseNYVEPRIA is not indicated for the mobilization of peripheral blood progenitorcells for hematopoietic stem cell transplantation.Please see full Prescribing Information, Patient Information, and Instructions for Use for NYVEPRIA at NyvepriaHCP.com.PP-PEG-USA-0011 2020 Pfizer Inc.All rights reserved. January 2021
Effective for dates of service on and after January 1, 2021, HCPCS code Q5122 may be used to report NYVEPRIA. 4 HCPCS Code1 Descriptor Q5122 Injection, pegfilgrastim-apgf, biosimilar, (nyvepria), 0.5 mg HCPCS Modifier2 Descriptor JB JA Subcutaneous administration Modifiers may be included on claims to provide additional information.