Transcription
AACN Advanced Critical CareVolume 17, Number 3, pp.272–283 2006, AACNReducing Foley Catheter Device Daysin an Intensive Care UnitUsing the Evidence to Change PracticeLaura Reilly, RN, BSN, CCRNPatty Sullivan, RN, BAIS, CICSharon Ninni, RN, BSN, CCRNDenise Fochesto, RN, MSN, CCRN, APN-CKaren Williams, BS, MT (ASCP) CICBrandee Fetherman, RN, BSN, CCRNABSTRACTThe prolonged use of indwelling urinarycatheters can lead to many complications, themost prevalent being urinary tract infections.These hospital-acquired infections can increase hospital costs, length of stay, and mortality rates. Evidence-based guidelines for theprevention of urinary tract infections are compared and discussed. Minimizing indwellingurinary catheter use is well-recognized in theliterature to reduce the risk of these infections. To decrease the incidence of catheterassociated urinary tract infections, the staff ofa 22-bed, mixed medical, surgical, andatheter-associated urinary tract infectionsC(UTIs) are the most common hospital-acquired infection and can lead to increasedlength of stays, mortality rates, and ultimatelyhigher hospital costs. The length of time foleycatheters remain in situ is directly related to increases in UTIs. This article describes a step-bystep clinical quality initiative to reduce UTIs byreducing foley catheter device days in an intensive care unit (ICU). A multidisciplinary teamwas assembled, and staff was engaged todevelop the plan. Criteria-based foley catheterguidelines, a decision-making algorithm, andan educational program were developed andimplemented. The strategy resulted in a signifi-trauma intensive care unit focused on reducing the number of foley catheter device days.A multidisciplinary team was convened tocreate an evidence-based plan. Staff nurseswere engaged in the development and implementation of the plan. Criteria-based foleycatheter guidelines, a decision-making algorithm, and a daily checklist were implemented that led to a significant reduction infoley catheter device days and a decrease incatheter-associated urinary tract infections.Keywords: critical care, urinary catheter,urinary tract infectionscant reduction in foley catheter device days anda decrease in catheter-associated UTIs. Thisproject supported an evidence-based qualityimprovement approach to change clinical practice and improve patient outcomes.Practice Problem: Incidence,Diagnosis, and Prevalence ofUrinary Tract InfectionsApproximately 30 million indwelling urinarycatheters are used in the United States eachLaura Reilly is ICU Coordinator, Morristown MemorialHospital, 80 Turner Street, Dover, NJ, 07801 (e-mail: laura.reilly@ahsys.org).272
V O L U M E 1 7 N U M B E R 3 J U LY – S E P T E M B E R 2 0 0 6F O L E Y C AT H E T E R Syear.1 This is estimated to be 15% to 25% ofall hospitalized patients.2 Urinary tract infections are the most prevalent hospital-acquiredinfection and are directly associated with theuse of indwelling urinary catheters, or foleycatheters. The literature estimates that UTIsaccount for 40% of all hospital-acquired infections, and that 80% of these infections aresecondary to a foley catheter.2 There are 2routes that allow bacteria to enter into thecatheterized patient. Intralumenal migrationallows passage through the inside of thecatheter tubing. Extralumenal migration allows passage in the space between the urethralmucosa and outside of the catheter tubing.3The National Nosocomial Infection Surveillance (NNIS) system is a national databasedeveloped by the Centers for Disease Controland Prevention (CDC) to benchmark infection rates of similar hospitals. The CDC classifies catheter-associated UTIs into 2 groups:symptomatic UTIs and asymptomatic bacteriuria (Table 1). The term catheter-associatedmeans that the patient had a foley catheter atthe time or within 7 days of the onset of theinfection.4NNIS criteria define the incidence ofcatheter-associated UTI by dividing the number of UTIs by the number of foley catheterdays and multiplying by 1000 (UTIs/1000 foley days). The NNIS benchmarks are published annually and are determined by calculating the pooled mean infection rates forsimilar participating critical care units. Thereare over 300 participating hospitals in theNNIS database. The pooled mean for medical/surgical ICUs in major teaching hospitalsin 2004 was 3.9 UTIs/1000 foley days.5The complication of a UTI can increase apatient’s hospital length of stay by 0.4 days foran asymptomatic UTI and 2.0 days for a sympomatic UTI.6 One recent cost analysis of UTIsestimated an additional expense ranging from 401 to 1,727 per UTI.7 Additional reportedestimates have been as high as 3,803 perinfection.8Other complications associated with theuse of foley catheters include urethritis, urethral strictures, hematuria, bladder perforation, catheter obstruction, and urosepsis.9Urosepsis is a life-threatening infectious complication, and its associated risk increases withprolonged indwelling urinary catheter usage.Urosepsis mortality rates are reported as highas 25% to 60%.10273Table 1: CDC Criteria for Diagnosis ofSymptomatic Urinary Tract Infectionand Unsymptomatic Bacteriuria4I. Symptomatic UTI must meet at least one of thefollowing criteria:1. Positive urine culture with 105 cfu/mL urinewith 2 or less bacterial species and 1 of thefollowing symptoms with no other recognizedcause:a. Fever ( 38 C)b. Frequencyc. Urgencyd. Dysuria or suprapubic pain at palpationapositive urine culture2. Two of the following symptoms with no otherrecognized cause: fever ( 38 C), frequency,urgency, dysuria or suprapubic pain atpalpation and one of the following findings:a. Evidence of leukocyte-esterase and/or nitrateb. Pyuria ( 10 leukocytes/mm3 or 3leukocytes/high power field ofuncentrifuged urine)c. Microscopic evidence of pathogen in Gramstain of uncentrifuged urined. Two urinary cultures with an identicaluropathogen 102 cfu/mL from correctlyobtained specimense. Pure culture with 105 cfu/mL afterappropriate antibiotic treatmentf. Physician diagnosis of a UTIg. Physician institutes appropriate therapy fora UTIII. Asymptomatic bacteriuria must meet at least oneof the following criteria:1. Patient has an indwelling urinary catheterwithin 7 days before the culture and patienthas a positive urine culture, that is 105cfu/mL with 2 or less bacterial species, andpatient has no fever ( 38 C), urgency,frequency, dysuria, or suprapubic tenderness.2. Patient has not had an indwelling urinarycatheter within 7 days before the first positiveculture and patient has had at least 2 positiveurine cultures, that is, 105 cfu/mL with 2 orless bacterial species and patient has no fever( 38 C), urgency, frequency, dysuria, orsuprapublic tenderness.UTI, urinary tract infection.
R E I L LY E T A LAACN Advanced Critical CareEvidence-based Practice:Strategies to Decrease UrinaryTract InfectionsEvidence-based guidelines for the care andmaintenance of foley catheters are publishedby a number of different groups,6,9,10,11 includingthe CDC,11 Joanna Brigg’s Institute,9 and theAssociation of Practitioners in Infection Control (APIC).6 Whereas these national guidelines are very similar, there are some differences in their recommendations (Table 2). Forexample, the CDC recommends properly securing the catheter to the leg, but JoannaBrigg’s Best Practice document does not address this issue. The CDC also recommendssterile technique during insertion, but JoannaBrigg’s states that sterile technique does not reduce the risk of UTI.12In an effort to base nursing practice on thebest supporting evidence at our institution,changes were implemented to decrease the incidence of catheter-associated UTIs. Productevaluation, practice issues, and education wereintegral to implementing change. There wereseveral studies that supported the use of silveralloy catheters for the reduction of catheter-associated UTIs13–16 and a meta-analysis of the research supported their use.17 Silver-alloycatheters were purchased and these cathetershave become the standard of care in this facility. Studies have shown the benefit of a closeddrainage system.18–20 Catheter kits were implemented to provide a closed system. To furtherensure a closed system, a bladder scanner waspurchased to scan the bladder and confirmwhether a decrease in urine output is due tocatheter blockage or reduced urine in thebladder.21 This minimizes unnecessary irrigation that would require a break in the closedsystem. Once these changes were initiated, securement devices were addressed. CDC guidelines strongly recommend the practice of properly securing the foley catheter to the leg.11Foley catheter leg-securing devices were usedto ensure an adequate securing system.Education was the next focus. There is variance regarding care practices of foleycatheters, and they are frequently not evidence-based.22,23 In one study,24 an estimated53.1% of patients with a catheter-associatedUTI had a “preventable mistake” in either theindications for or the management of thecatheter. Education regarding the care andmaintenance of foley catheters is recommended and has been shown to reducecatheter-associated UTIs.25 An educationalprogram was developed by the ICU nurseeducators in conjunction with the InfectionControl Department. A foley catheter educational video tape was purchased and all ICUnurses viewed the tape and completed aposttest. The video tape was kept in the conference room where the nurses could view it attheir convenience. All new hires were also required to view the tape.The accumulated results of these initiativesdecreased the incidence of catheter-associatedUTIs by approximately 50% from 1999 to2003. According to the NNIS,6 these ratesraised our unit to the top 50th percentile (thismeans that 50% of the hospitals reporting hadrates higher). Even though this was an acceptable improvement, further reductions in UTIincidence were desired. Recommendations inthe literature stress the importance of decreasing the unnecessary use of indwelling foleycatheters.6,11,26–28 Duration of catheterizationhas shown to be the major independent riskfactor for catheter-associated UTIs,29–30 andthere is some evidence that earlier removal ofurinary catheters is associated with a shorterlength of stay.31The ratio of foley catheter days to patientdays is part of the required data submitted tothe NNIS; these data are collected and calculated monthly. The NNIS comparative benchmark was approximately 82% to 84% (foleycatheter device days/patient days) and themonthly average for this ICU was 92% to100%. Previous attempts to decrease foleycatheter days through education, charge nursesurveillance, and the use of a daily goals sheet32in our ICU were unsuccessful. In an effort todecrease foley catheter-related complications(UTIs and urosepsis), our 22-bed, medical,surgical, and trauma ICU focused on reducingthe number of foley catheter device days in thecritical care setting.Team Development to ChangePracticeA multidisciplinary team is imperative to thesuccess of any quality improvement or evidence-based change in practice. The multifaceted complexity of a critically ill patient requires that all members caring for that patientenvision the same goals. All disciplines thatmay be affected by these practice changes mustbe consulted in the development phase, sothere is less resistance in the implementation274
V O L U M E 1 7 N U M B E R 3 J U LY – S E P T E M B E R 2 0 0 6F O L E Y C AT H E T E R STable 2: Comparison of Evidence-based Practice Guidelines to Decrease Urinary TractInfection RatesIndicatorPersonnelCenters for Disease Control and Prevention11and Association of Practitioners inInfection Control6 Guidelines*Only trained professionals/personnel,trained family members, trained patientsshould handle the catheter (Category I);Regular periodic inservices (Category II);Effective hand washing before and afterhandling (Category I).Catheter useUse only when necessary (Category I);Consider other catheters such as condomcatheters, suprapubic, and intermittentwhen appropriate (Category III);No specific recommendation as to thematerial used for catheter design.CatheterinsertionAseptic technique/sterile equipment(Category I);Smallest bore catheter possible (Category II);Joanna Briggs9 Guidelines*No Level I or II evidence-supportededucation; however, there were 3studies that indicated that continuousreinforcement through education mayreduce UTIs (Level III).One study suggests intermittentcatheterization of postop patientsmay reduce UTI, but further studiesneeded to support this (Level II);Silver impregnated catheters mayreduce UTI in certain patients, butfurther research needed and costeffectiveness is unclear (Level I).Aseptic technique does not reduce UTI(Level II);No recommendation of catheter bore;No recommendation for securement.Proper securement (Category I).Closed steriledrainageSterile, continuously closed drainagesystem (Category I);If break in aseptic technique ordisconnection, the catheter should bechanged (Category III).IrrigationIntermittent irrigation should be avoidedunless obstruction (Category II);Use aseptic technique to irrigate (Category I);Sterile, continuously closed drainagesystem recommended. but costshould be considered (Level I).Based on insufficient evidence, norecommendation of irrigation couldbe given.If frequent irrigation is needed, changecatheter (Category II).SpecimencollectionCollect small volumes from port usingaseptic technique (Category I);No discussion.Collect larger volumes from drainage bagusing aseptic technique (Category I).SpatialseparationInfected and uninfected patients should notshare the same room (Category III).No recommendation given.Urinary flowUnobstructed flow should be maintainedNo discussion*No kinks in tubing*Empty regularly with separate container(be sure spigot does not touch sides onnon-sterile container)*Keep drainage bags below bladder at alltimes (Category I)(continues)275
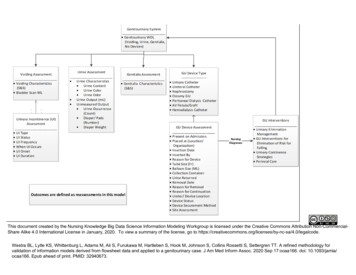
R E I L LY E T A LAACN Advanced Critical CareTable 2: Comparison of Evidence-based Practice Guidelines to Decrease Urinary TractInfection Rates (Continued )IndicatorMeatal careCenters for Disease Control and Prevention11and Association of Practitioners inInfection Control6 Guidelines*Routine daily cleaning with povidoneiodine solution or daily cleansing withsoap and water are not recommended(Category II);No further recommendations.Catheter changeintervalDo not change indwelling catheters atarbitrary fixed intervals (Category II).Joanna Briggs9 Guidelines*No specific recommendations otherthan “good personal hygiene”;Routine use of povidone-iodine,neomycin polymixin, beta-bacitracin,polyantibiotic products were notrecommended (Level I).Routine monthly catheter changessupported by one study (Level II);No recommendation given.*Centers for Disease Control and Prevention categories: Category 1: strongly recommended for adoption, Category 2: moderatelyrecommended for adoption, Category 3: weakly recommended for adoption; The Joanna Briggs Institute’s levels of evidence(recommendations are based only on randomized controlled trials): Level I: evidence obtained from a systematic review of all relevantrandomized controlled trials, Level II: evidence obtained from at leased one properly designed randomized controlled trial, Level III: (1)evidence obtained from well-designed controlled trials without randomization, (2) evidence obtained from well designed cohort or casecontrol analytic studies preferably from more than one center or research group, and (3) evidence obtained from multiple time series with orwithout the intervention. Dramatic results in uncontrolled experiments; Level IV: opinion of respected authorities, based on clinical experience,descriptive studies, or reports of expert committees.phase. A critical care nurse and infection control nurse were chosen as project leads by thechief nursing officer, ICU manager, and infection control manager, and the organization’sSix Sigma Department was consulted to guidethe project. Intensive care unit nurses, criticalcare physicians, nephrologists, urologists, infectious disease physicians, the ICU nursemanager, and representatives from the Infection Control Department were consulted todevelop criteria for foley catheter use in thispatient population.A thorough review of the literature regarding indications for the use of foley catheters inthe critical care setting and other patient populations was conducted.33–35 After reviewing theliterature with various nurses and physicians,criteria for appropriate foley catheter use weredetermined (Table 3). These ICU nurses andphysicians were doubtful that many patients inthis population could tolerate the removal offoley catheters. They were convinced that allthe ICU patients would meet these indicationsfor appropriate catheter use. The team leadersagreed that the results of this project may confirm these beliefs, but they persisted. The foleycatheter criteria were approved by the ICUCommittee and the Infection Control Committee and presented at other appropriate meetings, such as the medical and surgical businessmeetings and the ICU shared governance meeting. It was important to ensure that the goalswere communicated throughout the organization to reduce resistance during the pilot phase.Determining the Baseline forFoley Catheter Device DaysIn the early stages of the project, the measurement unit of foley catheter device days was defined. A device day began when a patient wasadmitted to the ICU with an indwelling foleycatheter intact or one was inserted in the ICU.A device day ended when the foley catheterwas discontinued in the ICU or the patient wasdischarged from the ICU with the foleycatheter intact. After conducting a sample sizecalculation, it was determined that 123 patientcharts were needed for preintervention data. Arandomized, convenience sample of 124 patient charts was gathered by the medicalrecord department. Exclusion criteria includedpatients who had expired in the ICU and patients with no foley catheter. A fishbone diagram (Figure 1) was developed by nursing staffduring small, informal brainstorming sessionsto determine possible causes of prolongedcatheterization in the ICU. Data were collectedto determine baseline foley catheter devicedays and to determine possible causal factorsof prolonged catheterization.276
V O L U M E 1 7 N U M B E R 3 J U LY – S E P T E M B E R 2 0 0 6F O L E Y C AT H E T E R Scollection tool be determined and the process ofdata collection be defined by comparing andevaluating data collected on a small pilot sample, in this case, 10 patient charts.After adequacy of the measurement systemwas determined, the charts were reviewed retrospectively through a manual data extractionprocess by the team leaders. The preintervention mean foley catheter device days was determined to be 4.72, with a standard deviation(SD) of 7.67 (n 124). Foley catheter deviceday were similar to the average ICU length ofstay in our unit, indicating that the majority ofthe patients had the continued use of thecatheter for most of the ICU stay.The next step was to analyze the multiplefactors that could contribute to the prolongeduse of foley catheters. During the baselinechart review, data was collected to determine:age of patient, physician service, delayedcatheter removal after a written physician’s order to discontinue, and length of time the patient met the criteria for the foley catheter (according to the criteria developed by this unit).A regression analysis found that 71.9%(P .05) (n 124) of the process variationwas due to the fact that the catheters were notbeing removed once criteria for removal wasmet. This was the only significant factorshown to contribute to prolonged foleycatheterization and became the focus of our efforts to further reduce the incidence of UTIs.Table 3: Criteria Indicating AppropriateFoley Catheter Use in a Medical/Surgical/Trauma Intensive Care UnitMajor Category 24-hour urine collection Epidural catheter Neurological head injury Skin breakdown in sacral area Spine X-rays not cleared Acute neurogenic bladder Clinical need for a foley, such as chemicallyparalyzed and sedated Crush injury Pelvic fractureSeparate Category Hemodynamically unstable needing accurateinput and output monitoring Hourly intake and output monitoring Inability to void Strict input and output monitoring required andpatient incontinentSurgical Category Gastric bypass surgery Renal surgeryThe data collection tool was created (Table 4)and evaluated by the team leaders to ensure thatthe measurement system was accurate. Therewere several problems encountered. The twodata collectors (the team leaders) were gathering the data from two different sources whichadded some variation to the data when compared. The infection control nurse was documenting the date and time of admission into theICU with the foley catheter by using the hospital computer system and the critical care nursewas using the ICU flowsheet. This caused variation in the data that could affect results. Afterthis was identified, consistency was established.There was also some variation when calculatingthe length-of-stay and device day measures. Inorder to obtain accuracy and consistency thedata collection tool was created in excel andformulas were used to provide accurate calculations. It is crucial that the accuracy of the dataEngaging the Staff to BrainstormSolutionsSigns were posted in the ICU conference roomto invite staff to be a part of the solution to decrease UTIs by participating in brainstormingsessions during several ICU luncheons. Staffcontinued to be doubtful that many of thecatheters in the ICU patients could be removedand they were concerned about the effects itmay have on their practice. Some of their concerns were patient focused, such as the potential increase in decubiti and the inability to accurately determine intake and output; somewere staff focused, such as the resistance tochange the current standard of practice in theICU; and some were process focused, such asthe inability to physically manage patientswithout catheters due to the current challengesthat their workload requires and the complexity of patient assignments.The team leaders promoted problem-solvingtechniques by working through these issues277
R E I L LY E T A LAACN Advanced Critical CareFigure 1: Fishbone diagram depicting possible causal factors for prolonged foley catheter devicedays.and allowing the nurses to discuss their concerns through continued brainstorming solutions. There were suggestions to have thecharge nurses, physicians, and/or residents conduct surveillance for catheter removal but thatwas rejected by the nursing staff. The nurses insisted that they have control of surveillance forcatheter removal and requested a decisionmaking algorithm to help guide their practice.It was decided to implement nurse-driven surveillance of criteria-based foley catheter guidelines through the use of a criteria-based foleycatheter checklist and a foley catheter decisionmaking algorithm. The checklist was createdby the nursing staff (Figure 2) and the algorithm was created by the team leaders with input from the multidisciplinary team (Figure 3).The nurses also suggested that an educationalprogram be developed and implemented. Theeducational program included complicationsrelated to prolonged catheterization and guidelines for appropriate foley catheter use. Theplans for education and project implementationwere presented for approval at the ICU andInfection Control Committee meetings. Institutional Review Board (IRB) approval wasobtained.An educational binder was created and utilized to educate ICU staff including nurses,residents, and physicians. The education wasTable 4: Foley Catheter Data Collection Tool Medical record number Age Service Date/time of intensive care unit (ICU) admission Date/time of ICU discharge ICU length of stay (calculated from 2 prior variables) Presence of foley on admission Date/time patient came to ICU with a foley orwhen foley inserted in the ICU Date/time of foley catheter removal or patienttransferred with foley in place Foley catheter device days Date/time patient met criteria for catheter removal Number of device days criteria met278
V O L U M E 1 7 N U M B E R 3 J U LY – S E P T E M B E R 2 0 0 6F O L E Y C AT H E T E R SICU Foley Catheter ChecklistDate:MR#:ICU room no.:Does your patient have a foley catheter?YNIs there an ICU order for the catheter?YNMedicalSurgicalWhich service is your patient on?TraumaWhich criteria for appropriate use of foley catheters does your patient meet?24-hour urine collectionEpidural catheterHead injurySkin breakdownSpine not clearAcute neurogenic bladderClinical need, ie, chemically paralyzed and sedatedCrush injuryPelvic fractureHemodynamically unstable needing accurate input and output (I&O)Hourly I&OInability to voidStrict I&O and incontinentGastric bypass surgeryRenal/urology surgeryColorectal surgery (questionable after 48-hour postop)Abdominal/pelvic surgery (questionable after 48 hour postop)If none of the above criteria are met, was an order for discontinuation offoley obtained? YNFIGURE 2: Intensive care unit foley catheter checklist.provided by the team leaders during multipleone-to-one in services for all shifts over a2-week period. These in services were conveniently located at the nurse’s station on a dailyinformal basis. A log was kept to ensure thatall staff completed the inservice. The education included risks associated with prolongedcatheterization such as UTIs, urosepsis, andmortality rates associated with urosepsis. Italso described appropriate indications for foley catheter use in the ICU through the use ofthe criteria-based foley catheter guidelines,and explained how to use the foley catheterdecision-making algorithm which was placedin the bedside binders for easy referral.The pilot was implemented upon completion of the staff education. A daily checklistfor every ICU patient with a foley catheter wascompleted by the nurses. The checklist functioned as a trigger to determine the necessity ofthe catheter. If a patient did not fit the criteriathe nurse would contact the physician to recommend catheter removal. The charge nursewas responsible for distribution and collectionof the daily checklistResults and Patient OutcomesAfter implementation of the pilot, a convenience sample of 83 charts were reviewed in thesame manner as the preintervention study. The279
R E I L LY E T A LAACN Advanced Critical CarePatients Without a Foley Catheter1. Assess patient and consider the following every 2 hours or more frequently as needed. Check for incontinence or offer bedpan. Assess for bladder distention. Turn and reposition and assess skin. Use a protective moisture barrier.2. If no voiding after 6 hours, utilize the bladder scanner to evaluate amount of urine inbladder: If greater than 300 mL or patient complains of discomfort and/or bladder distension onpalpation, call physician for straight catheter order X1. If less than 300 mL, reevaluate every 2 to 3 hours.3. If unable to void a second time, call MD for an order to insert a foley catheter.4. Call physician if no voiding after 12 hours.5. Consider twice a day or daily intermittent catheterization for patients with low urineoutput rather than a foley catheter.Figure 3: Decision-making algorithm to use for determining the appropriate use of a foley catheter.Reprinted with permission from the Morristown Memorial Hospital Intensive Care Unit, Morristown, NJ.D/C, discontinue.postintervention mean foley catheter devicedays were 2.98, with a SD of 3.17 (n 83).This was a decrease from preintervention foleycatheter device days of 4.72, with a SD of 7.67(n 124).A two-sample t-test was conducted on thepre- and postintervention means and a test forequal variance was completed for the pre- andpostintervention standard deviations. Thedata showed a statistically significant decrease280
V O L U M E 1 7 N U M B E R 3 J U LY – S E P T E M B E R 2 0 0 6F O L E Y C AT H E T E R Sdenominator (the NNIS calculates UTI ratesby calculating UTIs/1000 foley days). A decrease in device days could potentially makeeach UTI account for a higher NNIS rate. Wewere aware of the potential for this to occur, butfelt that the benefits of removing catheters outweighed the risks of potentially inflating infection rate percentages. It is also important to notethat the improvement achieved is even more impressive with this observation considered.Box 1: PATIENT RESPONSE TO FOLEYREMOVALNurse: “While assessing my patient at 0730, Irealized she did not meet the criteria for thefoley catheter so I initiated a call to the physicianto recommend catheter removal. I discontinuedthe catheter by 0830 and the patient couldn’tthank me enough. She was diagnosed with congestive heart failure and was capable of using abathroom and/or a bedpan and couldn’t understand the necessity of the catheter. The patientsaid, ‘The catheter was very uncomfortable andit was a tremendous relief to have it removed.’She was very grateful.”in the means (P 0.38; t-value –2.10; df 176; n 83). There was a 59% reduction inthe pre- and postintervention standard deviation, but the test for equal variance did notprove a statistically significant difference.However, the improvement has a practicalclinical significance that should be considered.Another important clinical consideration wasthat at preintervention, only 6% of the foleycatheters were removed before the patient wastransferred out of the ICU, but at postintervention, 20% of the foley catheters were removed prior to transfer out of the ICU. Whena patient leaves the ICU without the foleycatheter, chances are the foley catheter will notbe reinserted on the general patient care unit,and it is likely that hospital-wide, catheterassociated UTIs have also been affected.In the control phase of the project, a randomsam
in an Intensive Care Unit Using the Evidence to Change Practice AACN Advanced Critical Care Volume 17, Number 3, pp.272-283 . VOLUME 17 NUMBER 3 JULY-SEPTEMBER 2006 FOLEY CATHETERS 273 year.1 This is estimated to be 15% to 25% of . Extralumenal migration al-lows passage in the space between the urethral