Transcription
CongHrt Cover&Tabs9x1110/11/1112:55 PMPage 1CongenitalHeartDiseaseScreeningProgramToolkitA Toolkit for Implementing Screening 2nd EditionThis material is made possible by the Elsie and Marvin Dekelboum Family Foundation.
CongHrt Cover&Tabs9x1110/11/1112:56 PMPage 2AcknowledgementsThis material was made possible by a grant from theElsie and Marvin Dekelboum Family Foundation. The Children’s National Heart Instituteand Child Health Advocacy Institute would like to express sincere gratitudeto the Dekelboum Family Foundation for their support in the launch of this initiative.We would also like to thank the following Children’s National Medical Center departmentsand programs for their assistance in the development of this Toolkit: Language Services Legal Department Public Relations & Marketing Department Patient and Family Education Patient and Family Advisory Board Biomedical Engineering Nursing Staff DevelopmentSpecial acknowledgements are also given to the following: Mona Barmash, Children’s Heart Information Network Holy Cross Hospital
Children’s National Medical Center’sCongenital Heart Disease Screening ProgramTable of ContentsUser Agreement Section 1 – Program OverviewVisionPulse Oximetry Screening for Congenital HeartDisease: Who, What, When, Where, and Why?Opening LetterScreening RecommendationsLetter to ProvidersSupplies for ScreeningScreening Form Section 2 – Screener TrainingIn-Service Education Program ComponentsCongenital Heart Disease Screening Program:Education for ProvidersPerforming Pulse Oximetry with the InfantPatient: Education for ProvidersPulse Ox Probe Placement EducationKnowledge Assessment and Answer KeyCompetency Check ListTraining LogCHDSP PowerPoint Presentation Information Section 3 – Education for Parentsand GuardiansChecklist for Informing MothersFrequently Asked Questions (FAQs) for Patientsand FamiliesFrequently Asked Questions (FAQs) for Patientsand Families (Spanish)Congenital Heart Disease Screening Program:For Patients and FamiliesCongenital Heart Disease Screening Program:For Patients and Families (Spanish)CHD ResourcesCHD Resources (Spanish) Section 4 – Advocacy Section 5 – References and Resource Lists
User AgreementA. Terms and ConditionsPlease read this agreement in its entirety prior to use.The Congenital Heart Disease Screening ProgramToolkit (“Toolkit”) is designed to serve as a guide tohealthcare providers seeking to use pulse oximetry asa screening tool for critical congenital heart diseasein the newborn nursery. By utilizing this Toolkit, youagree to the terms and conditions that follow.B. DisclaimerRecommendations provided by Children’s NationalMedical Center are derived from a review of evidencebased literature on pulse oximetry screening forcritical congenital heart disease and outcomesof the clinical research study titled “Feasibilityof Implementation of Pulse Oximetry as a ScreeningTool for Critical Congenital Heart Disease in theNewborn Nursery.” Institutional Review Boardapproval was sought and obtained in this study.The information included in this Toolkit is forinformational and educational purposes only.Children’s National Medical Center makes reasonableefforts to ensure that the information provided iscomplete, and where appropriate, based on scientificevidence; however, Children’s National Medical Centermakes no assurances as to whether the providedinformation will at all times be current. Furthermore,this document does not reflect the best medicalpractice for all circumstances. Users of this Toolkitshould not substitute information contained forprofessional judgment, nor should they rely solely onthe information provided when rendering a diagnosisor choosing a course of medical treatment.Before undertaking implementation of any of thetactics discussed herein, users are advised to seekprofessional counsel on the issues raised byconsulting with medical staff and senior managersfor matters involving clinical practice and directpatient care.C. Intellectual PropertyChildren’s National Medical Center and any of itssubsidiaries/programs, www.childrensnational.org,the Children’s National Medical Center logo, and allother text and images contained within this documentare protected by the United States trademark andcopyright laws. All are considered to be property ofChildren’s National Medical Center, except as otherwiseidentified. All rights are reserved. Materials includedin this Toolkit may not be modified, reproduced,or displayed for any commercial or public purposewithout specific written permission from Children’sNational Medical Center. Any copyrights and trademarksthat are not the property of Children’s National MedicalCenter, which may also be referenced in this document,remain the property of their respective owners.D. LiabilityChildren’s National Medical Center does not warrantthe content of this document and specifically disclaims,to the fullest extent permitted by law, any and allwarranties, express or implied, of any kind or naturewhatsoever. Neither Children’s National MedicalCenter nor any of its subsidiaries/programs shall beliable under any circumstances for any claims, losses,or causes of action that may arise from the use of thisToolkit or the information contained in it.Congenital Heart Disease Screening Program 4
CongHrt Cover&Tabs9x1110/11/1112:56 PMPage 3Program OverviewVisionPulse Oximetry Screening forCongenital Heart Disease: Who,What, When, Where, and Why?Opening LetterScreening RecommendationsLetter to ProvidersSupplies for ScreeningScreening FormProgram OverviewSection 1
12:32 PMPage 4Program Overview‘‘This toolkit is an outstanding product that is the end result of muchconsideration and research, and will provide a straightforward,easy-to-use method to screen for cyanotic and serious forms ofcongenital heart disease in newborns. This program allows fora reliable, low-cost, high-throughput early detection program inthe nursery setting, reducing the risks of complications from delayeddiagnosis. I applaud the organizers and coordinators and hope thatnewborn pulse oximetry screening will quickly become widelyimplemented as standard of care.Charles I. Berul, MDChief, Division of CardiologyChildren's National Medical Center‘10/27/11‘CongHrt Cover&Tabs9x11Through our expereince with research and helping hospitalsto start CCHD screening programs in their nurseries we havelearned a lot about best-practice for implementation. We hopethat you find the contents in this toolkit helpful and weapplaud you for raising the bar in your nurseries to improvethe outcomes of patients and families with CCHD.Elizabeth A. Bradshaw, MSN, RN, CPNClinical Program Coordinator, Cardiac Research
Vision: All infants with critical congenital heart disease are detected before leaving the nursery.Who, What, When, Where, and Why?What is congenital heart disease Why is pulse ox used to screen for CHD?and pulse oximetry?Congenital heart disease (CHD) is the mostcommon birth defect. Infants with CHD haveabnormal structure to their heart which createsabnormal blood flow patterns. Approximatelyeight of every 1,000 infants born have a form ofCHD. Some forms of CHD cause no or very fewproblems in the health, growth, and developmentof the infant. However, critical CHD can bringa significant risk of morbidity and mortality ifnot diagnosed soon after birth. Failing to detectcritical CHD while in the newborn nursery maylead to critical events such as cardiogenic shockor death. Survivors who present late are atgreater risk for neurologic injury and subsequentdevelopmental delay.Pulse oximetry, or “pulse ox,” is a simple,non-invasive and painless test that is usedto measure the percent oxygen saturation ofhemoglobin in the arterial blood and the pulserate. Pulse ox was invented in the 1970s and isnow widely used and accepted in clinical care;it is often thought to be a basic vital sign.Pulse ox can help to identify infants with critical CHDthat may have low levels of oxygen in their blood.Pulse ox screening may help diagnose critical CHDbefore an infant becomes sick.Who should be screened?All infants should be screened.When should screening be performed?Pulse ox screening should be performed before dischargefrom the nursery, after the infant turns 24 hours of age.If the infant was born prematurely, screening should beperformed when medically appropriate. If early dischargeis planned, screening should occur as late as possible.Whereshould pulse ox screeningbe performed?Pulse ox screening should be performed while the infant isin the nursery, before he or she goes home. The pulse oxtest should be performed on the right hand and one foot.Congenital Heart Disease Screening Program 7Program OverviewVision
Program OverviewDear Provider,Thank you for your interest in the Congenital Heart Disease Screening Program(CHDSP). We are excited to provide you with the resources that you will need toimplement the CHDSP in your newborn nursery. The components of this programhave been assembled by Children’s National Medical Center. Components arebased on a review of current literature and recommendations, outcomes forresearch on best-practice for implementation, and our experience helpingnurseries to implement screening.Background and SignificanceGerard R. Martin, MD, FAAP,FACCJoseph L. Wright, MD, MPHAs you know, congenital heart disease (CHD) is the most common birth defectand may be detected during either the prenatal or postnatal period. Prenataltesting, utilizing ultrasound technology, is an important early screening mechanismfor life threatening heart disease; however it has been shown that diagnosis maybe made in only 23 percent of pregnancies or 11 percent of live births. Detectionduring the postnatal period is currently done by either physical examination, orby detection of symptoms during the first 24 hours of life. These methods haveproven to be successful in identifying only 50 percent of infants with CHD.Failing to detect critical CHD while in the newborn nursery may lead to seriousevents such as cardiogenic shock or death. Survivors who present late are atgreater risk for neurologic injury and subsequent developmental delay. Earlydetection of critical CHD can potentially improve the prognosis and decreasethe mortality and morbidity rate of affected infants. Pulse oximetry has beeninvestigated and proven to be successful in detecting some forms of critical CHDin the newborn nursery.Health and Human Services Secretary Kathleen Sebelius endorses the inclusionof screening for critical CHD in the recommended uniform screening panel.The American Heart Association, American Academy of Pediatrics and AmericanCollege of Cardiology also support newborn pulse oximetry screening for criticalCHD. In January, 2011 the Health Resource Service Administration’s AdvisoryCouncil on Heritable Diseases in Newborns and Children hosted a workshop todiscuss implementation recommendations surrounding screening. The outcomeof this meeting included a screening protocol based on the most currentevidence. This protocol is reflected in the recommendations which follow.Congenital Heart Disease Screening Program 8
Toolkit MaterialsThis screening program adds pulse oximetry testing ofthe right hand and one foot to routine testing performedon all infants. The test should be performed after theinfant turns 24 hours of age, or when medicallyappropriate if the infant was born prematurely. It isrecommended that pulse oximetry screening be donein conjunction with other standard-of-care newbornscreening that requires the infant be at least 24 hoursof age, such as metabolic or hearing screening.This toolkit will provide you with the initial resourcesneeded to start the CHDSP in your newborn nursery.The toolkit includes information regarding theimplementation of the CHDSP, resources for trainingindividuals responsible for screening, and resourcesfor educating families. In addition, all materials forthe education of families are provided in both Englishand Spanish. Education materials are evidence-based.If the newborn’s oxygen saturation is 95% in eitherextremity, with a 3% difference between the two he orshe will be considered to pass the screening test and noadditional evaluation will be required unless signs orsymptoms of CHD are present.If the newborn’s oxygen saturation is 90% in eitherthe hand or foot he or she should be immediatelyreferred for additional evaluation.The toolkit also includes simple ways that parents,families, healthcare professionals, and others canbecome advocates for patients with CHD.We are excited to work with you to implement theCHDSP in your newborn nursery. There is thepotential to save the lives and improve outcomesof many babies with CHD.If the oxygen saturations are 95% in both the handand foot or there is a 3% difference between thetwo on three measures each separated by one hour thenewborn should be referred for additional evaluation.Sincerely,Gerard R. Martin, MD, FAAP, FACCSenior Vice PresidentCenter for Heart, Lung, and Kidney DiseaseJoseph L. Wright, MD, MPHSenior Vice PresidentChild Health Advocacy InstituteCongenital Heart Disease Screening Program 9Program OverviewOverview of CHDSP Screening Guidelines
Program OverviewCongenital Heart Disease Screening Program:Screening RecommendationsSection 1: Recommendations forImplementation Planning10. Establish guidelines for education of and communication with parents and guardians before andafter screening.1. Designate a program director and coordinator tofacilitate planning and implementation of thescreening program.11. Establish plans for surveillance and reporting ofprogram results and outcomes.2. Establish an interdisciplinary team of organizationalleadership and management, physicians, registerednurses, nursing assistants, and ancillary staff toparticipate in the planning process.3. Schedule several planning sessions to facilitateeducation, communication, brainstorming, conflictresolution, and decision making.4. Ensure that the organization’s public relations andmarketing department is involved in communicationplanning and efforts.5. Discuss and establish a clear, complete, and conciseevidence-based policy and procedure for screeningmethods and guidelines, including documentationand reporting of normal and abnormal results.6. Discuss plan for management and evaluation ofinfants requiring further evaluation if pediatriccardiology services are not available on site.7. Establish guidelines for parents or guardians whowish to decline screening.12. Birthing facilities at high altitudes may requirerevised protocols.Section 2: Recommendations forParental Education1. Establish a plan to inform parents of the screeningprogram prior to delivery and screening of theinfant through:a. prenatal classes and tours provided by thehospital,b. information on hospital’s web site, andc. written materials available in the obstetricsand gynecology clinics, labor and delivery,and maternity suites.2. Provide education in both written and verbalmethods; written materials should be easy to readand understand, and should not contain excessivemedical language that may be confusing to parents.3. Provide written materials in English and Spanish;consider additional languages based on patientpopulation that is served and use an interpreterwhen appropriate.8. Research the accuracy and reliability of pulseoximetry equipment. Choose a vendor withmotion-resistant equipment.9. Establish guidelines for informing, educating, andtraining providers and staff participating in and/oraffected by implementation of the screening program.4. Include program contact information on allcommunication to provide mothers the opportunityto seek additional information and clarificationprior to delivery.5. Inform parents of the right to decline screening.Congenital Heart Disease Screening Program 10
1. Inform and educate all hospital and communityproviders who work in the newborn nursery,neonatal intensive care unit, postpartum unit,and pediatrics that will be affected by the screeningprogram prior to implementation.- Consider sending out a letter of program intentseveral weeks prior to implementation.- Provide program contact information to allowproviders to seek additional information andclarification.2. Provide a Grand Rounds session for the educationof hospital and community providers.3. Request time at department meetings to informand educate hospital and community providersprior to implementation.4. Following implementation, provide frequent updatesto hospital and community providers on screeningprogram progress and outcomes at departmentmeetings or through written communication.Section 4: Recommendations forScreener Training1. Provide all training prior to implementation of thescreening program by an individual who hasparticipated in the planning process.a. Examples include the unit’s nurse manager orassistant nurse manager, the nurse educator,the program coordinator, or a registered nursewho played an active role in the planningprocess.2. Recommended components of the in-serviceeducation program include:a. CHDSP PowerPoint Presentation- Includesinformation on background and significancefor screening and CHDSP screening methodsand recommendations and accessible throughwww.childrensnational.org/pulseoxb. Demonstration of correct and safe use of pulseoximetry equipment in obtaining an accurateinfant reading by trainer or representativefrom pulse oximeter manufacturer.c. Completion of knowledge assessment quiz.d. Opportunity to practice pulse oximetryscreening.3. Require that all individuals who will be performingthe screening test complete the in-service educationprogram.4. Require that all individuals who will be performingthe screening test complete the knowledgeassessment quiz with a passing score of greaterthan or equal to 90 percent, with remediation ofall questions answered incorrectly.5. Require that all individuals who will be performingthe screening test demonstrate proficiency inperforming pulse oximetry and knowledge ofscreening guidelines through completion of definedcompetencies prior to participation. Require thatthey renew competencies on an annual basis.6. Provide “booster” sessions quarterly to provide anopportunity to re-educate staff and answer anyquestions.7. Ensure that all new employees receive trainingprior to participation in screening program methods.8. Provide staff with regular updates on outcomes ofscreening to maintain engagement.Congenital Heart Disease Screening Program 11Program OverviewSection 3: Recommendations for Educatingand Informing Providers
Program OverviewSection 5: Recommendations for Screening1. Pair pulse oximetry screening with anotherstandard-of-care screening performed following24 hours of age, such as metabolic or hearingscreening. If early discharge is planned, screeningshould occur as late as possible.2. Consider assigning one or two nursing assistants orregistered nurses to pulse oximetry screening on adaily basis.a. If possible, provide continuity by schedulingone screener to conduct screening on severalcontinuous days.3. Conduct screening in quiet area with parentpresent to soothe and comfort the infant.4. If possible, conduct screening while the infant isawake, quiet, and calm.5. Do not attempt to perform pulse oximetry on aninfant while he or she is sleeping, crying or coldas oxygen saturations may be affected.6. If using disposable pulse ox probes, use one cleanprobe for each infant screened. If reusable probesare being used, clean probe as instructed bymanufacturer prior to and following screening.Dirty probes may decrease the accuracy of areading or transmit infection.a. The physician or nurse practitioner caring forthe infant does not need to be notified.b. The infant does not require additional cardiacevaluation in the newborn nursery unlessindicated.10. If the pulse ox reading is 90% in either thehand or foot, the infant should be immediatelyreferred to his or her physician for additionalevaluation.11. If the oxygen saturations are 95% in both thehand and foot or there is a 3% differencebetween the two on three measures each separatedby one hour the newborn should be referred foradditional evaluation.a. The infant’s physician or nurse practitionershould be notified.b. Infectious and pulmonary pathology shouldbe excluded.c. If cause of hypoxemia is not clear anechocardiogram and cardiology consultationshould be obtained before discharge to ruleout congenital heart disease.d. Further evaluation should be ordered at thediscretion of the physician or nursepractitioner caring for the infant.Section 6: Recommendations for Follow-Up7. Perform pulse oximetry on the right hand and onefoot after 24 hours of age; measurements shouldbe taken in parallel or one after another. If infantwas born prematurely, perform screening whenmedically appropriate.8. Ensure that all readings are accurate by using pulseoximetry equipment confidence indicators.1. Establish guidelines for documentation andcommunication of results and plan of care(if necessary) with infant’s parents andpediatrician.2. Establish guidelines for individuals performingscreening if asked questions by parents.9. If the oxygen saturation is 95% in eitherextremity, with a 3% difference between the twothe infant will “pass” the screening test and noadditional evaluation will be required unless signsor symptoms of CHD are present.Congenital Heart Disease Screening Program 12
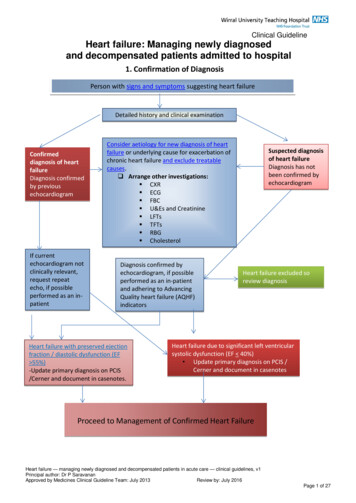
Screening Protocol DiagramPulse Ox on Right Hand (RH) and One Foot After 24 Hours of AgePulse Ox 95% (RH or Foot) andDifference of 3% Between RH and FootPulse Ox 95% (both RH & foot) orDifference of 3% Between RH and FootFAILPASSRepeat Pulse Ox in 1 HourNormal Newborn CareFAILRepeat Pulse Ox in 1 HourFAILClinical AssessmentRH Application SiteREMINDER ALGORITHMFOR SCREENERSASSESSMENT OF BABIES WITH FAILING SATURATIONS Confirm that the infant is at least 24hours of age and eligible for screening. Help the parent to warm and calm theinfant in a quiet and peaceful environment. Describe the pulse ox testto the parent. Select a site on the right handand one foot that is clean and dry.Foot Application Site1. Babies with saturation of 90 % in RH or foot should haveimmediate assessment.2. Babies with Failing Saturations:- Clinical Assessment- Infectious and Pulmonary pathology should be excluded- Complete echocardiogram- If symptomatic, referral to Pediatric Cardiology immediately- If asymptomatic referral to Pediatric Cardiology in timely manner Place the pulse ox probe andperform the pulse ox test.Congenital Heart Disease Screening Program 13Program OverviewCongenital Heart Disease Screening Program:
Congenital Heart Disease Screening Program:Template for Provider Letterof Program IntentThe following template is a suggestion for notifying providers of implementation of screening.Congenital Heart Disease Screening Program 14
We are excited to inform you that we will be implementingthe Congenital Heart Disease Screening Program (CHDSP) inour newborn nursery. The CHDSP involves the use of pulseoximetry as a screening tool for critical congenital heart disease(critical CHD) in the newborn nursery. The components of thisprogram have been assembled by Children’s National MedicalCenter and are based on a review of current literature on pulseoximetry screening for critical CHD as well as outcomes ofresearch on best-practice for implementation. This letter willinform you of the background and significance of pulseoximetry screening for critical CHD and provide an overviewof recommended guidelines.Background and SignificanceAs you know, CHD is the most common birth defect and maybe detected during either the prenatal or postnatal period.Prenatal testing, utilizing ultrasound technology, is an importantearly screening mechanism for life threatening heart disease;however it has been shown that diagnosis may only be made in23 percent of pregnancies or 11 percent of live births. Detectionduring the postnatal period is done by physical examination ordetection of symptoms during the first 24 hours of life and issuccessful in identifying only 50 percent of infants with CHD.Failing to detect critical CHD while in the nursery maylead to critical events such as cardiogenic shock or death.Survivors who present late are at greater risk for neurologicinjury and subsequent developmental delay. Early detectionof critical CHD can potentially improve the prognosis anddecrease the mortality and morbidity rate of affected infants.Health and Human Services Secretary Kathleen Sebeliusendorses the inclusion of screening for critical CHD in therecommended uniform screening panel. The American HeartAssociation, American Academy of Pediatrics and AmericanCollege of Cardiology also support pulse oximetry screeningof newborns.Overview of CHDSP Screening GuidelinesThe CHDSP adds pulse oximetry to routine testing after 24hours of life to detect critical CHD. It is recommended thatpulse oximetry screening be done in conjunction withanother standard-of-care newborn screening that requiresthe infant be at least 24 hours of age.All newborns should be screened. Pulse oximetry should beperformed on the right hand and one foot. If the newborn’soxygen saturation is 95% in either extremity, with a 3%difference between the two he or she will be considered topass the screening test and no additional evaluation will berequired unless signs or symptoms of CHD are present.If the newborn’s oxygen saturation is 90% in either the handor foot he or she should be immediately referred for additionalevaluation.If the oxygen saturation is 95% in both the hand and foot orthere is a 3% difference between the two on three measureseach separated by one hour the newborn shouldbe referred for additional evaluation. All future decisionsregarding care of newborns with lower than expected saturationswill be made at the discretion of the physician or nursepractitioner caring for the infant. It is recommended that anechocardiogram be obtained to rule out structural abnormalitiesfor newborns with abnormal pulse oximetry readings.We are asking that you work with us to implement theCHDSP in our newborn nursery. We are excited to havethe opportunity to work with you to implement a screeningprogram that has the potential to save the lives and improveoutcomes for many of our babies. Please feel free to contactus with any additional questions or concerns.Sincerely,(SIGNATURE)Congenital Heart Disease Screening Program 15Program OverviewDear Provider,
Program OverviewCongenital Heart Disease Screening Program:Supplies for Screening Pulse Oximeters At least one motion-tolerant pulse oximeter tobe used for screening One motion-tolerant pulse oximeter for back-up Rolling Cart for Supplies Infant Disposable or Reusable Pulse Ox Sensors If using disposable sensors, one disposablesensor for every infant screened If using reusable sensors, one reusable sensorfor each pulse oximeter. Also consider additionalreusable sensors for back-up– Disinfecting agent recommended by pulseoximetry equipment manufacturer One disposable wrap per infant screened tosecure sensor to hand or foot Dedicated individual to perform screening Data Collection Forms One for every infant screened Red Heart-Shaped Stickers One red heart-shaped sticker for every infantwho has been screened Blankets for warming the infant and blockingextraneous light A parent for comforting infant during screeningCongenital Heart Disease Screening Program 16
Program OverviewPLACE LABEL OR WRITE-IN INFORMATIONMedical Record #Patient Name: LastDate of BirthFirst//Congenital Heart Disease Screening Program:Screening FormAge at Initial Screening:hoursInitial Screening:TimePulse Ox Saturation of Right HandPulse Ox Saturation of FootDifference (right hand – foot)%%% Pass FailSecond Screening (1 hour following initial screen if fail initial screen)TimePulse Ox Saturation of Right Hand%Pulse Ox Saturation of Foot%Difference (right hand – foot)% Pass FailThird Screening (1 hour following second screening if fail second screen)TimePulse Ox Saturation of Right Hand%Pulse Ox Saturation of Foot%Difference (right hand – foot)% Pass Fail* If pulse ox saturation is 90% in either the hand or foot the infant’s MD or NP must be notified immediately.“Fail must be checked”.* If pulse ox saturations are 95% in both the hand and foot or there is a 3% difference between the two onthree measures each separated by one hour the MD or NP must be notified. “Fail must be checked.”* If pulse ox saturations are 95% in either extremity, with a 3% difference between the two the readingis expected for an infant. “Pass” should be checked”.Screener’s Name:Screener’s Signature:Date:Congenital Heart Disease Screening Program 17//
Program OverviewNotesCongenital Heart Disease Screening Program 18
CongHrt Cover&Tabs9x1110/11/1112:56 PMPage 5Section 2Screener TrainingIn-Service Education Program ComponentsCongenital Heart Disease Screening Program:Education for ProvidersPulse Ox Probe Placement EducationKnowledge Assessment and Answer KeyCompetency Check ListTraining LogCHDSP PowerPoint PresentationScreener TrainingPerforming Pulse Oximetry with the InfantPatient: Education for Providers
10/11/1112:56 PMPage 6Screener Training‘‘CongHrt Cover&Tabs9x11Pulse oximetry screening for critical congenital heart disease willsave lives. I would do anything to go back in time and have thissimple test performed on my daughter. She might be with ustoday." Olivia Easley, advocate and mother of Veronica Easley whopassed away at 7 weeks old from critical congenital heart disease.Olivia Easley with daughter Veronica
In-Service EducationProgram Componentsand RecommendationsThe following is an overview of educational tools and components that may beused to educate staff who will be directly involved in screening implementation.Educational tools discussed ar
Congenital Heart Disease Screening Program 4 A. Terms and Conditions Please read this agreement in its entirety prior to use. The Congenital Heart Disease Screening Program Toolkit ("Toolkit") is designed to serve as a guide to healthcare providers seeking to use pulse oximetry as a screening tool for critical congenital heart disease