Transcription
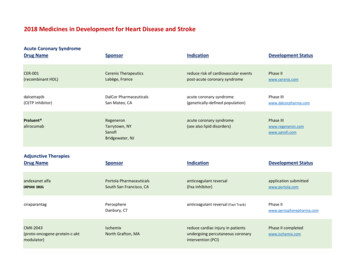
2018 Medicines in Development for Heart Disease and StrokeAcute Coronary SyndromeDrug NameSponsorIndicationDevelopment StatusCER-001(recombinant HDL)Cerenis TherapeuticsLabège, Francereduce risk of cardiovascular eventspost-acute coronary syndromewww.cerenis.comdalcetrapib(CETP inhibitor)DalCor PharmaceuticalsSan Mateo, CAacute coronary syndrome(genetically-defined population)www.dalcorpharma.comPraluent alirocumabRegeneronTarrytown, NYSanofiBridgewater, NJacute coronary syndrome(see also lipid disorders)SponsorIndicationDevelopment StatusPortola PharmaceuticalsSouth San Francisco, CAanticoagulant reversal(Fxa inhibitor)www.portola.comPerosphereDanbury, CTanticoagulant reversal (Fast Track)IschemixNorth Grafton, MAreduce cardiac injury in patientsundergoing percutaneous coronaryintervention (PCI)Adjunctive TherapiesDrug Nameandexanet alfaORPHAN aktmodulator)Phase IIPhase IIIPhase IIIwww.regeneron.comwww.sanofi.comapplication submittedPhase IIwww.perospherepharma.comPhase II completedwww.ischemix.com
Adjunctive TherapiesDrug NameSponsorIndicationKengreal cangrelorChiesi USACary, NCpartial or complete obstruction ofsystemic to pulmonary artery shuntin patients at risk for thrombosis(neonates)levosimendan IV(R-enantiomer of simendan)Tenax TherapeuticsMorrisville, NClow cardiac output syndromeprevention in high risk cardiac surgery(see also pulmonary vascular disease)www.tenaxthera.comArrhythmiasDrug NameSponsorIndicationDevelopment Statusbucindolol(beta blocker/vasodilator)ARCA BiopharmaWestminster, COprevention of symptomatic atrialfibrillation/atrial flutter in patientswith heart failure (Fast Track)Phase II/IIIwww.arcabio.combudiodarone(calcium, potassium, sodiumchannel antagonist)Espero BiopharmaJacksonville, FLatrial fibrillation, ventricular tachycardiain patients with implantablecardioverter defibrillatorswww.esperobio.cometripamil(calcium channel antagonist)Milestone PharmaceuticalsQuebec, Canadatreatment of symptomatic acuteepisodes of paroxysmal flecainide inhalation(sodium channel antagonist)InCarda TherapeuticsBrisbane, CAparoxysmal atrial fibrillationPhase IORPHAN DRUGDevelopment StatusPhase Iwww.chiesiusa.comPhase IIIPhase IIPhase IIwww.ioncardatherapeutics.com
ArrhythmiasDrug NameSponsorIndicationCardiome PharmaVancouver, Canadaatrial fibrillationORPHAN DRUGCardiomyopathyDrug NameSponsorIndicationARRY-797(p38 mitogen-activatedprotein kinase inhibitor)Array BioPharmaBoulder, COLMNA-related dilated cardiomyopathyCAP-1002(allogeneic cardiosphere-derivedstem cell therapy)Capricor TherapeuticsBeverly Hills, CADuchenne muscular dystrophy-relatedcardiomyopathy(see also heart attack)MYK-461/SAR439152(myosin inhibitor)MyoKardiaSouth San Francisco, CASanofiBridgewater, NJsymptomatic, obstructive tructive lant IVORPHAN DRUGMYK-491(allosteric activator of myosin)MyoKardiaSouth San Francisco, CASanofiBridgewater, NJDevelopment StatusPhase III completedwww.cardiome.comDevelopment StatusPhase IIwww.arraybiopharma.comdilated cardiomyopathyPhase I/IIwww.capricor.comPhase IIPhase IPhase Iwww.myokardia.com
CardiomyopathyDrug NameSponsorIndicationPB-1046(VPAC2 selective agonist)PhaseBio PharmaceuticalsMalvern, PADuchenne muscular dystrophyinduced cardiomyopathy(see also heart failure, pulmonaryvascular disease)PfizerNew York, NYtransthyretin amyloid cardiomyopathyPhase III(Fast Track)www.pfizer.comCoronary Artery DiseaseDrug NameSponsorIndicationDevelopment Statusapabetalone(BET protein inhibitor)ResverlogixSan Francisco, CAsecondary prevention of majoradverse cardiac events (MACE) inpatients with coronary artery disease,type 2 diabetes and low HDLcholesterolPhase IIIAZD5718(5-lipoxygenase activatingprotein inhibitor)AstraZenecaWilmington, DEcoronary artery diseaseBrilinta ticagrelorAstraZenecaWilmington, DEcardiovascular outcomes in patientswith coronary artery disease andtype 2 diabetes without a previoushistory of myocardial infarctionor strokeORPHAN DRUGtafamidis meglumine(amyloid inhibitor)Development StatusPhase Iwww.phasebio.comORPHAN DRUGwww.resverlogix.comPhase IIwww.astrazeneca.comPhase IIIwww.astrazeneca.com
Coronary Artery DiseaseDrug NameSponsorIndicationDevelopment StatusCOMBO Stent (anti-CD34 mAb/sirolimus drugeluting stent)OrbusNeichFort Lauderdale, FLprevention of coronary arteryrestenosis in patients with coronaryartery diseasewww.orbusneich.comCVBT-141H(fibroblast growth factor-1)CardioVascular BioTherapeuticsDallas, TXsevere coronary heart diseaseDP9(progesterone transdermal)DimeraPortland, ORmyocardial ischemiaGenerxalferminogene tadenovec(recombinant angiogenicgene therapy)AngioneticsSan Diego, CArefractory angina due to myocardialischemia (cardiac microvascularinsufficiency) (Fast Track)MEDI5884(cholesterol modulator)MedImmuneGaithersburg, MDcoronary heart diseaseMEDI6012(LCAT therapy)MedImmuneGaithersburg, MDcoronary artery diseaseridaforolimus eluting coronary stent(device drug combination)MedinolTel Aviv, Israelcoronary artery restenosisumirolimus(drug-eluting stent)Biosensors InternationalSingaporecoronary artery restenosisin clinical trialsPhase IIwww.cvbt.comPhase II completedwww.dimera.netPhase IIIwww.angionetics.comPhase IIwww.medimmune.comPhase IIwww.medimmune.comPhase IIIwww.medinol.comPhase IIIwww.biosensors.com
Coronary Artery DiseaseDrug NameSponsorIndicationVBL TherapeuticsModi’in, IsraelatherosclerosisHeart AttackDrug NameSponsorIndicationBVI-007(thrombopoietin inhibitor)BioVascularSan Diego, CAprevention of myocardial infarctionin patients who have had a previouscardiovascular event(see also stroke)CAP-1002(allogeneic cardiosphere-derivedstem cell therapy)Capricor TherapeuticsBeverly Hills, CAmyocardial infarction(see also cardiomyopathy)www.capricor.comCSL 112(apolipoprotein A-I infusion therapy)CSL BehringKing of Prussiareduction of recurrent cardiovascularevents following myocardial infarctionwww.cslbehring.comEntresto sacubitril/valsartanNovartisEast Hanover, NJpost-acute myocardial infarction(see also heart failure)www.novartis.comFDY-5301Faraday PharmaceuticalsSeattle, WAmyocardial infarctionVB-201Development StatusPhase IIwww.vblrx.comDevelopment StatusPhase Iwww.biovascularinc.comPhase I/IIPhase IIPhase IIIPhase IIwww.faradaypharma.com
Heart AttackDrug NameSponsorIndicationDevelopment Statusmesenchymal stem cell therapy(ischemic-tolerant stem cells)Stemedica Cell TechnologiesSan Diego, CAacute myocardial infarction(see also heart failure, stroke)www.stemedica.comMultiStem allogeneic stem cell therapyAthersysCleveland, OHacute myocardial infarction(see also stroke)www.athersys.comREC-01(CD26 antigen inhibitor)RecardioSan Francisco, CAacute myocardial infarctionRGN-352 injectable(thymosin beta-4)RegeneRx BiopharmaceuticalsRockville, MDacute myocardial infarction(see also stroke)www.regenerx.comTF0023(aspirin pro-drug)Techfields PharmaDover, DEheart attack recovery(see also stroke)www.tfpharma.comHeart FailureDrug NameSponsorIndicationDevelopment StatusAdipoCell adipose-derived autologousstem cell therapyU.S. Stem CellMiami, FLcongestive heart failureAironitesodium nitrite inhalationSavara PharmaceuticalsAustin, TXdiastolic heart failurePhase I completedPhase IIPhase Iwww.recardio.euPhase I completedPhase IIPhase I/IIwww.us-stemcell.comPhase IIwww.savarapharma.com
Heart FailureDrug NameSponsorIndicationAMG 986(apelin receptor agonist)AmgenThousand Oaks, CAheart failureAR-06(hydralazine/isosorbide dinitratecontrolled release)Arbor PharmaceuticalsAtlanta, GAheart failureAZD4831(myeloperoxidase modulator)AstraZenecaWilmington, DEheart failure with a preservedejection fractionwww.astrazeneca.comBAY1142524(chymase inhibitor)Bayer HealthCare PharmaceuticalsWhippany, NJleft ventricular dysfunction(heart s SquibbPrinceton, NJchronic heart failurePhase IBMS-986231(nitroxyl donor)Bristol-Myers SquibbPrinceton, NJacute heart failureBR6819(vasopressin receptor antagonist)Bayer HealthCare PharmaceuticalsWhippany, NJheart failureCardiALLO (allogenic culture expandedmesenchymal stem cells derivedfrom bone marrow)BioCardiaSan Carlos, CAheart failureDevelopment StatusPhase Iwww.amgen.comPhase IIIwww.arborpharma.comPhase IPhase IIwww.bms.comPhase IIwww.bms.comPhase Iwww.pharma.bayer.comPhase Iwww.biocardia.com
Heart FailureDrug NameSponsorIndicationNovartisEast Hanover, NJchronic stable heart failureStealth BioTherapeuticsNewton, MAacute heart failure, heart failure withpreserved ejection fraction, heartfailure with reduced ejection fractionwww.stealthbt.comEntresto sacubitril/valsartanNovartisEast Hanover, NJheart failure with preservedejection fraction(see also heart attack)www.novartis.comEZR104(valsartan extended release)Ezra PharmaLittle Rock, AKheart failure(see also hypertension)Farxiga dapagliflozinAstraZenecaWilmington, DEworsening heart failure or cardiovascular death in patients withchronic heart failure(see also other)FuroscixscPharmaceuticalsBurlington, MAchronic heart failureCLR325elamipretidesubcutaneous furosemideDevelopment StatusPhase IIwww.novartis.comPhase IIPhase IIIapplication submittedwww.ezrapharma.comPhase IIIwww.astrazeneca.comapplication submittedwww.scpharmaceuticals.comdecompensated heart failurePhase II/IIIwww.scpharmaceuticals.com
Heart FailureDrug NameSponsorIndicationGSK2798745(TRPV4 antagonist)GlaxoSmithKlineResearch Triangle Park, NCheart failureInjectafer ferric carboxymaltoseDaiichi SankyoBasking Ridge, NJLuitpold PharmaceuticalsShirley, NYheart failure with iron deficiency andwith a reduced ejection fractionIW-1973(soluble guanylate cyclase agonist)Ironwood PharmaceuticalsCambridge, MAheart failure with preserved ejectionfractionixmyelocel-T(stem cell therapy)VericelCambridge, MAheart failure due to ischemicdilated cardiomyopathy (Fast Track)www.vcel.comJardiance empagliflozinBoehringer IngelheimRidgefield, CTEli LillyIndianapolis, INheart failure with preserved 0(non-viral gene therapyexpressing SDF-1)Juventas TherapeuticsCleveland, OHheart failure (Fast Track)(see also peripheral vascular disease)www.juventasinc.comLIK066(SGLT 1/2 inhibitor)NovartisEast Hanover, NJpatients with type 2 diabetes andheart failurewww.novartis.comDevelopment StatusPhase IIwww.gsk.comPhase IIIwww.dsi.comPhase IIwww.ironwoodpharma.comPhase II completedORPHAN DRUGPhase IIIPhase IIPhase II
Heart FailureDrug NameSponsorIndicationSarfez PharmaceuticalsVienna, VAchronic congestive heart failuremesenchymal stem cell therapy(ischemic-tolerant stem cells)Stemedica Cell TechnologiesSan Diego, CAchronic heart failure(see also heart attack, stroke)MPC-150-IM(human mesenchymal stemcell therapy)MesoblastNew York, NYadvanced heart failurelong-acting loop diureticDevelopment StatusPhase IIwww.sarfezpharma.comPhase IIwww.stemedica.comPhase IIIwww.mesoblast.comend-stage heart failurePhase IIwww.mesoblast.comMyoCell muscle-derived autologousstem cell therapyU.S. Stem CellSunrise, FLsevere heart damage in heart failureneladenoson bialanate(adenosine A1 receptor agonist)Bayer HealthCare PharmaceuticalsWhippany, NJchronic heart failureNeucardinrecombinant human neuregulin-1 betaZensun USASan Diego, CAchronic heart failureomecamtiv mecarbil(cardiac myosin activator)CytokineticsSouth San Francisco, CAAmgenThousand Oaks, CAheart failurePhase II/IIIwww.us-stemcell.comPhase IIwww.pharma.bayer.comPhase IIwww.zensunus.comPhase IIIwww.amgen.com
Heart FailureDrug NameSponsorIndicationOpsumit macitentanActelion PharmaceuticalsSouth San Francisco, CAheart failure with preserved ejectionfraction and pulmonary arterialdisease(see also pulmonary vascular disease,other)PB-1046(VPAC2 selective agonist)PhaseBio PharmaceuticalsMalvern, PAheart failure with preserved or reducedejection fraction(see also cardiomyopathy,pulmonary vascualr disease)PL-3994 NPR-A(cyclic natriuretic peptidereceptor A agonist)Palatin TechnologiesCranbury, NJheart failureREC-02(chemokine CXCL12 modulator)RecardioSan Francisco, CAcongestive heart failureRT-100(gene therapy)Renova TherapeuticsSan Diego, CAcongestive heart failure (Fast Track)RT-400(peptide therapy)Renova TherapeuticsSan Diego, CAacute decompensated heart failureSAR440181(DCM1 myosin activation)SanofiBridgewater, NJpost acute heart failureDevelopment StatusPhase IIwww.actelion.comPhase IIwww.phasebio.comPhase IIwww.palatin.comPhase Iwww.recardio.euPhase IIIwww.renovatherapeutics.comPhase IIwww.renovatherapeutics.comPhase Iwww.sanofi.com
Heart FailureDrug NameSERCA 2a gene therapySponsorIndicationDevelopment StatusTheragene PharmaceuticalsSan Diego, CANYHA class III/IV heart failurePhase II(Fast Track)www.theragenepharma.comTD-0714(neprilysin inhibitor)Theravance BiopharmaSouth San Francisco, CAheart failurePhase ITD-1439(neprilysin inhibitor)Theravance BiopharmaSouth San Francisco, CAheart failureularitide(atrial peptide agonist/diuretic)CardiorentisZug, Switzerlandacute heart failure (Fast Track)vericiguat(sGC stimulator)Bayer HealthCare PharmaceuticalsWhippany, NJchronic heart failureHypertensionDrug NameSponsorIndicationDevelopment StatusAF-130(purinergic P2X3 receptorantagonist)MerckKenilworth, NJhypertensionPhase I completedwww.merck.comaprocitentan(dual endothelin receptorantagonist)Idorsia PharmaceuticalsAllschwil, SwitzerlandJanssen BiotechHorsham, PAresistant hypertension managementPhase IIwww.theravance.comPhase Iwww.theravance.comPhase IIIwww.cardiorentis.comPhase com
HypertensionDrug NameSponsorIndicationatorvastatin/aspirin/ramipril fixeddose combination (polypill)Ferrer InternacionalBarcelona, SpainhypertensionB244(bacteria-based therapeutic)AOBiomeCambridge, MAhypertensiondigoxin immune FabORPHAN DRUGAMAG PharmaceuticalsWaltham, MAEdarbi azilsartan medoxomilArbor PharmaceuticalsAtlanta, GAhypertension (pediatric)EZR104(valsartan extended release)Ezra PharmaLittle Rock, AKhypertension(see also heart failure)IONIS-AGT-LRX(LICA-conjugated antisense drug)Ionis PharmaceuticalsCarlsbad, CAtreatment resistant hypertensionKBP-5074(mineralocorticoid receptor antagonist)KBP BiosciencesPrinceton, NJhypertension in advanced chronickidney diseaseKIT-302(amlodipine/celecoxib fixed-dosecombination)Kitov PharmaceuticalsTel Aviv, Israeltreatment of hypertension andosteoarthritis pain simultaneouslyDevelopment StatusPhase IIwww.ferrer.comPhase IIwww.aobiome.comsevere preeclampsiaPhase II/III(Fast Track)www.amagpharma.comPhase IIIwww.arborpharma.comapplication submittedwww.ezrapharma.comPhase IIwww.ionispharma.comPhase I/IIwww.kbpbiosciences.comapplication submittedwww.kitovpharma.com
HypertensionDrug NameSponsorIndicationDevelopment StatusKybiqoolmesartan medoxomil/rosuvastatinfixed-dose combinationStocosilIndustry, CAhypertension and hyperlipidemia(see also lipid disorders)www.stocosil.comLHW090NovartisEast Hanover, NJresistant hypertensionQGC001(brain aminopeptidase A inhibitor)Quantum GenomicsParis, FrancehypertensionRMJH-111b(magnesium citrate)RMJ HoldingsAshburn, VAessential hypertensionPhase I/II completedLipid DisordersDrug NameSponsorIndicationDevelopment Status1-MNA(active metabolite of niacin)CortriaBoston, MAPharmenaLodz, PolandhyperlipidemiaAKCEA-ANGPTL3-LRX(ANGPTL3 protein inhibitor)Akcea TherapeuticsCambridge, MArare hyperlipidemiasAKCEA-APO(a)-LRX(ligand conjugated antisense drug)Akcea TherapeuticsCambridge, MAhyperlipoproteinemia withsignificant cardiovascular riskPhase IIPhase IIwww.novartis.comPhase IIwww.quantum-genomics.comPhase II completedwww.pharmena.euPhase IIwww.akceatx.comPhase IIwww.akceatx.com
Lipid DisordersDrug NameSponsorIndicationAKCEA-APOCIII-LRX(ligand conjugated antisense drug)Akcea TherapeuticsCambridge, MAhypertriglyceridemiaAMG 899(CETP inhibitor)AmgenThousand Oaks, CAdyslipidemiaARI-3037MO(GPR109A/GPR109B receptor agonist)Arisaph PharmaceuticalsBoston, MAdyslipidemia, hypertriglyceridemiabempedoic acid (oral)Esperion TherapeuticsAnn Arbor, MIlowering elevated low-densitylipoprotein (LDL) cholesterol,cardiovascular disease risk reductionwww.esperion.combempedoic acid/ezetimibefixed-dose combination (oral)Esperion TherapeuticsAnn Arbor, MIlowering elevated LDL cholesterolPhase IIICaPre long-chain Omega-3 phospholidAcasti PharmaQuebec, CanadahypertriglyceridemiaEpanova omega-3 carboxylic acidsAstraZenecaWilmington, DEhigh-risk for cardiovascular eventsin patients with persistent hypertriglyceridemia and low HDL cholesterolevinacumab (REGN1500)(ANGPTL-1 antibody)RegeneronTarrytown, NYhomozygous familialhypercholesterolemiaDevelopment StatusPhase IIwww.akceatx.comPhase IIwww.amgen.comPhase IIwww.arisaph.comPhase IIIwww.esperion.comPhase II completedwww.ascatipharma.comPhase IIIwww.astrazeneca.comPhase IIwww.regeneron.com
Lipid DisordersDrug NameSponsorIndicationGemphire TherapeuticsLivonia, MIsevere hypertriglyceridemia,homozygous familialhypercholesterolemia, heterozygousfamilial hypercholesterolemiainclisiran(RNAi therapeutic)The Medicines CompanyParsippany, NJhypercholesterolemiaK-312Kowa Research InstituteMorrisville, NCdyslipidemiaKybiqoolmesartan medoxomil/rosuvastatinfixed-dose combinationStocosilIndustry, CAhypertension and hyperlipidemia(see also hypertension)www.stocosil.comKynamro mipomersenKastle TherapeuticsChicago, ILheterozygous .comMGL-3196(liver-directed thyroid hormonereceptor beta agonist)Madrigal PharmaceuticalsWest Conshohocken, PAheterozygous pemafibrate (K-877)(selective PPAR-alpha modulator)Kowa Research InstituteMorrisville, NCdyslipidemiaPhase IIIPF-06427878(DGAT2 inhibitor)PfizerNew York, NYhyperlipidemiagemcabene(acetyl-CoA carboxylase inhibitor/lipoprotein A inhibitor)ORPHAN DRUGDevelopment StatusPhase IIwww.gemphire.comPhase IIIwww.themedicinescompany.comPhase Iwww.kowaus.comPhase IIPhase II completedPhase IIwww.kowaus.comPhase Iwww.pfizer.com
Lipid DisordersDrug NameSponsorIndicationDevelopment StatusRegeneronTarrytown, NYSanofiBridgewater, NJhomozygous familialhypercholesterolemia(see also acute coronary ockville, MDUniversity of PennsylvaniaPhiladelphia, PAhomozygous familialhypercholesterolemiawww.regenxbio.comZydus CadilaGujarat, Indiadyslipidemia ( 500 mg/dL)SC401(docosahexaenoic acid/eicosapentaenoic acid)SancilioRiveria Beach, FLsevere hypertriglyceridemiaVK-2809(thyroid hormone receptorbeta agonist)Viking TherapeuticsSan Diego, CAhypercholesterolemiavolanesoren(antisense ApoCIII inhibitor)Akcea TherapeuticsCambridge, MAfamilial chylomicroenemiasyndromePraluent alirocumabORPHAN DRUGRGX 501(personalized gene therapy)ORPHAN DRUGsaroglitazarORPHAN DRUGPhase IIIPhase I/IIPhase IIwww.zyduscadila.comPhase IIwww.sancilio.comPhase IIwww.vikingtherapeutics.comapplication submittedwww.akceatx.com
Peripheral Vascular DiseaseDrug NameSponsorIndicationACP-01(angiogenic cell precursor therapy)HemostemixCalgary, Canadacritical limb ischemiaALD-301(bone marrow-derived adult stem cells)AldagenDurham, NCperipheral arterial occlusive disordersASCT01(autologous stem cell therapy)LifecellsIrvine, CAcritical limb ischemiaautologous stem cell therapyBioTech HoldingsRichmond, CAischemia associated with diabetesBAY1193397(AR alpha-2c receptor antagonist)Bayer HealthCare PharmaceuticalsWhippany, NJperipheral arterial diseasebeperminogene perplasmid(HGF plasmid)AnGes USABethesda, MDMitsubishi Tanabe PharmaOsaka, JapanCVBT-141C(fibroblast growth factor-1)CardioVascular BioTherapeuticsDallas, TXperipheral artery diseasehuman plasma-derived fibrinolysinGrifols TherapeuticsLos Angeles, CAacute peripheral arterial occlusionORPHAN DRUGDevelopment StatusPhase IIwww.hemostemix.comPhase II completedPhase I/IIwww.lifecellsllc.comin clinical trialsPhase IIwww.pharma.bayer.comcritical limb ischemiaPhase III(Fast Track)www.anges.co.jpPhase IIwww.cvbt.comPhase IIwww.grifols.com
Peripheral Vascular DiseaseDrug NameSponsorIndicationDevelopment StatusJVS-100(non-viral gene therapy)Juventas TherapeuticsCleveland, OHperipheral artery disease (Fast Track)(see heart failure)www.juventasinc.comLiprostin alprostadil (liposomalcontrolled release)AngioSomaMontgomery, TXperipheral artery diseaseLLG783(LDL receptor antagonist mAb)NovartisEast Hanover, NJintermittent claudication,peripheral arterial disorderMABp1(IL-1-alpha/IgG1 mAb)XBiotechAustin, TXperipheral vascular disease (Fast Track)MultiGeneAngioperipheral arterial disease cell therapyMultiGene Vascular SystemsHaifa, Israelperipheral arterial diseaseNVS therapy(peripheral vascular disorderdrug-device therapy)Alucent MedicalSalt Lake City, UTperipheral vascular diseasePLX-PAD cell therapyPluristem TherapeuticsHaifa, Israelcritical limb ischemia (Fast Track)Symic BioEmeryville, CAperipheral arterial diseaseSB-030(inflammation mediator modulator)Phase IIPhase IIwww.antisomainfoPhase IIwww.novartis.comPhase IIwww.xbiotech.comPhase I/IIwww.mgvs.co.ilPhase Iwww.alucentmedical.comPhase IIIwww.pluristem.comPhase IIwww.symic.bio
Peripheral Vascular DiseaseDrug NameSponsorIndicationTV1001SR(sodium nitrate oral)TheravascCleveland, OHperipheral arterial diseaseVM202(donaperminogene seltoplasmid)ViroMedSeoul, South Koreaperipheral artery diseasevonapanitase(recombinant human elastase)Proteon TherapeuticsWaltham, MADevelopment StatusPhase IIwww.theravasc.comPhase IIIwww.viromed.co.krperipheral artery diseasePhase III(Fast Track)www.proteontherapeutics.comBayer HealthCare PharmaceuticalsWhippany, NJJanssenRaritan, NJinfrainguinal peripheral arterialdisease(see also stroke, thrombosis, dicationDevelopment StatusABI-009(sirolimus albumin boundnanoparticle)AadiPacific Palisades, CApulmonary arterial hypertensionPhase Iambrisentan/tadalafilfixed-dose combinationGlaxoSmithKlineResearch Triangle Park, NCpulmonary arterial hypertensionORPHAN DRUGXarelto rivaroxabanPulmonary Vascular DiseaseDrug NamePhase IIIPhase I completedwww.gsk.com
Pulmonary Vascular DiseaseDrug NameSponsorIndicationDevelopment Statusbardoxolone methyl(Nrf2 activator)Reata PharmaceuticalsIrving, TXconnective tissue disease associatedwith pulmonary arterial hypertension(CTD-PAD)www.reatapharma.comORPHAN DRUGpulmonary hypertension-interstitiallung disease (PH-ILD)beraprost 314dLung BiotechnologySilver Spring, MDpulmonary arterial hypertensionCAM2043(treprostinil controlled release)CamurusLund, Swedenpulmonary arterial hypertensionGeNOsylnitric oxide inhalationGeNOAtlanta, GApulmonary arterial hypertensionGSK2256098(FAK inhibitor)GlaxoSmithKlineResearch Triangle Park, NCpulmonary arterial hypertensionGSK2586881(recombinant human angiotensinconverting enzyme)GlaxoSmithKlineResearch Triangle Park, NCpulmonary arterial hypertensionINOpulsenitric oxide inhalationBellerophon TherapeuticsWarren, NJpulmonary arterial hypertensionORPHAN DRUGPhase IIIPhase IIwww.reatapharma.comPhase IIIwww.lungbiotechnology.comPhase I/IIwww.camurus.comPhase II completedwww.genollc.comPhase I completedwww.gsk.comPhase I completedwww.gsk.comPhase IIIwww.bellerophon.com
Pulmonary Vascular DiseaseDrug NameSponsorIndicationINS1009(inhaled, sustained-releasenebulized prodrug formulationof treprostinil)InsmedBridgewater, NJpulmonary arterial hypertensionKAR5585(tryptophan hydroxylase 1 inhibitor)Karos PharmaceuticalsNew Haven, CTpulmonary arterial hypertensionLetairis ambrisentanGilead SciencesFoster City, CAGlaxoSmithKlineResearch Triangle Park, NCpulmonary arterial hypertension(pediatric)levosimendan IV(R-enantiomer of simendan)Tenax TherapeuticsMorrisville, NCpulmonary hypertension due to heartfailure with preserved ejection fraction(see also adjunctive therapies)LIQ861(inhalation)Liquidia TechnologiesMorrisville, NCpulmonary arterial hypertensionOpsumit macitentanActelion PharmaceuticalsSouth San Francisco, CApulmonary arterial hypertension(pediatric), portopulmonaryhypertension(see also heart failure, other)Development StatusPhase Iwww.insmed.comPhase IORPHAN DRUGORPHAN DRUGPhase IIwww.gilead.comwww.gsk.comPhase IIwww.tenaxthera.comPhase IIIwww.liquidia.comchronic thromboembolic pulmonaryhypertensionPhase IIIwww.actelion.comPhase IIwww.actelion.com
Pulmonary Vascular DiseaseDrug NameSponsorIndicationDevelopment StatusOreniLeft treprostinilUnited TherapeuticsSilver Spring, MDpulmonary arterial hypertensionassociated with left ventriculardiastolic dysfunction (WHO group 2)www.unither.comPB-1046(VPAC2 selective agonist)PhaseBio PharmaceuticalsMalvern, PApulmonary arterial hypertension(see also cardiomyopathy,heart failure)www.phasebio.comArena PharmaceuticalsSan Diego, CApulmonary arterial hypertensionPhase IIRemoSynch implantable system for Remodulin (treprostinil)United TherapeuticsSilver Spring, MDpulmonary arterial hypertensionRemUnity treprostinil continuous subcutaneousvia pre-filled pumpUnited TherapeuticsSilver Spring, MDpulmonary arterial hypertensionsGC activator 2(soluble guanylate cyclase)Bayer HealthCare PharmaceuticalsWhippany, NJpulmonary hypertensionsildenafilPfizerNew York, NYpersistent pulmonary arterialhypertension (neonates)ORPHAN DRUGralinepag(prostacyclin receptor agonist)Phase IIIPhase IIwww.arenapharm.comORPHAN DRUGapplication submittedwww.unither.comPhase IIIwww.unither.comPhase Iwww.pharma.bayer.comPhase IIIwww.pfizer.com
Pulmonary Vascular DiseaseDrug NameSponsorIndicationVIVUSCampbell, CApulmonary arterial hypertensionORPHAN DRUGTrevyenttreprostinil patch pumpSteadyMed TherapeuticsSan Ramon, CApulmonary arterial hypertensionTysuberprost esuberaprost in combinationwith Tyvaso (treprostinil)United TherapeuticsSilver Spring, MDpulmonary arterial hypertension(decrease morbidity and mortality)Tyvaso treprostinilUnited TherapeuticsSilver Spring, MDpulmonary arterial hypertensionassociated with chronic obstructivepulmonary disease (WHO group 3)www.unither.comTyvaso-ILD treprostinilUnited TherapeuticsSilver Spring, MDpulmonary arterial hypertensionassociated with idiopathicpulmonary fibrosis (WHO group 3)www.unither.comUptravi selexipag intravenousActelion PharmaceuticalsSouth San Francisco, CApulmonary arterial hypertension(switch from oral to intravenous)www.actelion.comVentaProstepoprostenol inhalationAerogen PharmaGalway, Irelandpulmonary hypertensionPhase IItacrolimusDevelopment StatusPhase IIwww.vivus.comapplication submittedwww,steadymed.comORPHAN DRUGPhase IIIwww.unither.comPhase IIIPhase IIIPhase IIIwww.areogen.com
StrokeDrug NameSponsorIndicationDevelopment Status3K3A-APC(recombinant human wild-typeactivated protein C)ZZ BiotechHouston, TXmoderate to severe ischemic strokePhase IIwww.zzbiotech.comBIIB093 (glibenclamide IV)BiogenCambridge, MAlarge hemispheric infarction(severe form of ischemic stroke)ORPHAN DRUGPhase IIwww.biogen.com(Fast Track)BVI-007(thrombopoietin inhibitor)BioVascularSan Diego, CAprevention of thrombotic strokein patients who have had a previouscardiovascular event(see also heart attack)Phase Iclazosentan(endothelin receptor antagonist)Idorsia PharmaceuticalsAllschwil, Switzerlandcerebral vasospasm associated withaneurysmal subarachnoid hemorrhagewww.idorsia.comCN-105(apolipoprotein E agonist)AegisCNRaleigh, NCintracerebral hemorrhagePhase IIDM199(recombinant human kallikrein-1)DiaMedica TherapeuticsMinneapolis, MNacute ischemic strokePhase IIDS-1040(TAFIα inhibitor)Daiichi SankyoBasking Ridge, NJacute ischemic strokewww.biovascularinc.comPhase IIIORPHAN DRUGwww.diamedica.comPhase Iwww.dsi.com
StrokeDrug NameSponsorIndicationDevelopment StatusEG-1962(nimodipine microparticles)Edge TherapeuticsBerkeley Heights, NJaneurysmal subarachnoid hemorrhage(intraventricular delivery) (Fast Track)www.edgetherapeutics.comaneurysmal subarachnoid hemorrhage(intracisternal delivery)www.edgetherapeutics.comPhase IIIORPHAN DRUGPhase ILT-3001(antioxidant/free radical scavenger)Lumosa TherapeuticsTaipei, Taiwanacute ischemic strokePhase IMAA868(anti-FXI mAb)NovartisEast Hanover, NJstroke prevention in atrial fibrillationmesenchymal stem cell therapy(ischemic-tolerant stem cells)Stemedica Cell TechnologiesSan Diego, CAischemic stroke(see also heart attack, heart failure)www.stemedica.comMultiStem allogeneic stem cell therapyAthersysCleveland, OHischemic stroke (Fast Track)(see also heart attack)www.athersys.comNA-1(PSD-95 antagonist)NoNOToronto, Canadastroke with endovascular interventionnatalizumab(alpha-4 integrin inhibitor)BiogenCambridge, MAacute ischemic strokewww.lumosa.com.twPhase IIwww.novartis.comPhase IIPhase IIPhase IIIwww.nonoinc.caPhase IIwww.biogen.com
StrokeDrug NameSponsorIndicationNVX-208(dodecafluoropentane nanoemulsion)NuvOx PharmaTucson, AZacute ischemic strokePF-05230907(factor Xa protein replacement)PfizerNew York, NYintracerebral hemorrhagePradaxa dabigatran etexilateBoehringer Ingelheim PharmaceuticalsRidgefield, CTsecondary stroke prevention inpatients with embolic stroke ofundetermined source(see also thrombosis)RGN-352 injectable(thymosin beta-4)RegeneRx BiopharmaceuticalsRockville, MDstroke(see also heart attack)www.regenerx.comsalvinorin A(opioid kappa receptor agonist)Neurokappa TherapeuticsPhiladelphia, PAcerebral vasospasmPhase ISB623(modified bone marrow derivedmesenchymal stem cells)SanBioMountain View, CASunovion PharmaceuticalsMarlborough, MAischemic strokestroke polyfunctional therapeuticHSRx BiopharmaceuticalTucson, AZstrokeDevelopment StatusPhase I/IIwww.nuvoxpharma.comPhase Iwww.pfizer.comORPHAN DRUGPhase IIIwww.boehringer-ingelheim.comPhase I completedPhase IIwww.sanbio.comwww.sunovion.comPhase Iwww.hsrxbiopharmaceutical.com
StrokeDrug NameSponsorIndicationDevelopment StatusTF0023(aspirin pro-drug)Techfields PharmaDover, DEischemic stroke(see also heart attack)www.tfpharma.comXarelto rivaroxabanBayer HealthCare PharmaceuticalsWhippany, NJJanssenRari
(ischemic-tolerant stem cells) San Diego, CA (see also heart attack, stroke) www.stemedica.com MPC-150-IM Mesoblast advanced heart failure Phase III (human mesenchymal stem New York, NY www.mesoblast.com cell therapy) end-stage heart failure Phase II www.mesoblast.com MyoCell U.S. Stem Cell severe heart damage in heart failure Phase II/III











