Transcription
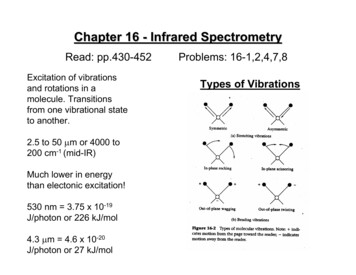
Chapter 16 - Infrared SpectrometryRead: pp.430-452Excitation of vibrationsand rotations in amolecule. Transitionsfrom one vibrational stateto another.2.5 to 50 µm or 4000 to200 cm-1 (mid-IR)Much lower in energythan electonic excitation!530 nm 3.75 x 10-19J/photon or 226 kJ/mol4.3 µm 4.6 x 10-20J/photon or 27 kJ/molProblems: 16-1,2,4,7,8Types of Vibrations
Dipole Changes During Vibrations andRotationsA molecule must undergo a net change in dipole moment as a consequenceof its vibrational and rotational motion in order to absorb IR radiation. Onlythen can the alternating electric field of the radiation interact with themolecule and produce a change in the amplitude of one of its motions.When two charges, q and q-, are separated by a distance, R, then a dipolemoment exists. Directed from negative toward positive end.p (Debye, C-m) q R H-Cl--O C O -
Classical and Quantum Mechanical Pictureof Two Atoms in a Bond Vibratingk E hυm h µ2πF -kyE (1/2)ky21k1k(m1 m2)υm 2π µ 2π m m1 2
An Absorption ExampleA linear, symmetric molecule!O C OPredicted # of vibrations for a linear molecule 3N-5Predicted # of vivbrations for a non-linear molecule 3N-6CO23 x 3 – 5 4 normal modesSymmetric (inactive)- Asymmetric (2330 cm-1 (4.3 µm))-Degenerate bending motions (667 cm-1 (15 µm))
InstrumentationSources (weakly intense!!)1. Nernst glower (rare earth oxides2. Glowbar (SiC rod)Detectors (must be stable, have fastresponse time and be highlysensitive)1. Thermal transducers (temperaturechanges)2. Thermocouples (junction of twometals with a resistance thatchanges with temperature)3. Pyroelectric (changes intemperature cause polarization inmaterial to change4. Photoconductors (incident photonscause charge separationinternally)Instruments must havegood focusing andcollection optics!!
Go back and read pp. 206-212!!
Advantages of Fourier TransformSpectrometers Very high light throughput (fewer optical components) Jaquinot advantage. High resolution ( 0.01 cm-1). All wavelengths of light reach the detectorsimultaneously multiplex advantage. Fast speed and improved sensitivity (S/N ratios).
Typical FTIR Spectrometer
Typical Background Spectrum of Air
Chapter 17: Applications of InfraredSpectroscopyRead: pp. 404-421Problems: noneStructural identification of molecules quantitative information!
Identification of Structural Features
Quantitative InformationWider slitwidths leadsto widerbandwidths.This results innonlinearBeer’s LawbehaviorA εbC log Psolvent/Psolution
Sample Handling Solvents water and alcohol are seldom used as they absorbstrongly and attack cell window materials. No solvent istransparent through-out the entire mid-IR region.Cells NaCl or KBr often used as a transparent material –sample holder.Samples gases, liquids or solids. Pelleting (1 part sample: 1parts KBr, press to make a transparent pellet) and mulls(dispersing solid in mineral oil).
Principles of FTIR Spectroscopy In FTIR analyses, Infrared light from the light source passes through a Michelsoninterferometer along the optical path.The Michelson interferometer comprises a beam splitter, moving mirror, and fixedmirror. The light beam split into two by the beam splitter is reflected from the movingmirror and fixed mirror, before being recombined by the beam splitter. As the moving mirror makes reciprocatingmovements, the optical path difference to the fixedmirror changes, such that the phase differencechanges with time. The light beams are recombinedin the Michelson interferometer to produceinterference light. The intensity of the interference light is recorded inan interferogram, with the optical path differencerecorded along the horizontal axis.
Principles of Diffuse Reflectance MethodMeasurement of chemicals adhering to a surface - PowdersK is the absorption coefficient, and S is the scatteringcoefficient. In practice, the comparative reflectancer with respect to a standard powder such as KBr orKCl, of which K is near zero (0) in the actualmeasurement rangeSpectrum of solid caffeineShimadzu website
High Sensitivity Reflection MeasurementA reflection method is required to measure substances adhered to or appliedto a material that does not permit light transmission, such as a metal sheet.Path lengthThin FilmOnly the parallel polarized light affects theabsorption by the sample so using apolarizer for measurements increases theapparent peak size. Information on thesample orientation can also be acquired, asonly functional groups with a perpendiculardipole moment with respect to the metalsheet are measured. However, suchincreases in sensitivity are available onlywith a metal substrate.Shimadzu website
High Sensitivity Reflection MeasurementA reflection method is required to measure substances adhered to or appliedto a material that does not permit light transmission, such as a metal sheet.sp3 C-H stretching modesSpectrum of a 25 Å-thick organic film on a Au surface.Shimadzu website
Attenuated Total ReflectanceEnables samples to be examined directly in the solid or liquid statewithout further preparation.Diamond, Si, Ge (high refractive index)Penetration depth 0.5-2 μmShimadzu website
Fluorescence and FTIR MicroscopyDetectorUseful for generating spatial maps of “vibrational modes”. For example,tissue analysis, polymer homogeneity, pharmaceutical quality, forensics.Jasco website
Principles of FTIR Spectroscopy In FTIR analyses, Infrared light from the light source passes through a Michelson interferometer along the optical path. The Michelson interferometer comprises a beam splitter, moving mirror, and fixed mirror. The light beam split i










