Transcription
Practical of ClinicalHematologyCollected and prepared byIbtisam H. Al AswadMohammed M. LaqqanAida Z. Al MasriMedical Technology DepartmentIslamic University-Gaza2008١
ﺒﻴﺔ ﻟﻄ ﻟﻴﻞﺍ ﺎ ﻟﺘﺤ ﻗﺴﻢﺍ ﻠﻮﻡ ﻟﻌ ﻠﻴﺔﺍ ﻛ ﺰﺓ ﺎﻣﻌﺔﺍﻹﺳﻼﻣﻴﺔﻏ ﺍﳉ ﺎﻕ ﻭﺻﻒﺍﳌﺴ ﻠﻲ ﻤ مﻋ ﺪ ﻟ ا ﻢ ﻠ ة : ﻋ د ﺎ ﻤ ﻟ ا ﻢ اﺳ ة MEDI 3116 : د ﺎ ﻤ ﻟ ﻢا ﻗ ر ل 2008 : و اﻷ ﻞ ﻔﺼ ﻟ ا ﺔ( ﯿ ﻠ ﻤ ﺎتﻋ ﺎﻋ ة ) ٣ ﺳ ﺪ ﻤ ﺘ ﻌ ةﻣ ﺪ اﺣ ﺔو ﺎﻋ ﺔ : ﺳ ﯿ ﻟ ﺎ ﻤ ﻹﺟ ا ﺎت ﺎﻋ ﻟﺴ ا د ﺪ ﻋ م . ﺪ ﻟ ا ﻢ ﻠ ﻞﻋ ﯿ ﻠ ﺔﻓﻲﺗﺤ ﯿ ﻟ ﺎ ﻟﺤ ا ﺮق ﻠﻄ ﯿﻖﻟ ﺒ ﺗﻄ ﺔو ﯿ ﻠ ﻤ ﻌ ﻟ ا رب ﺎ ﺠ ﺘ ﻠ ﻟ ﺔ اﺳ ر ﺎق : د ﻤﺴ ﻟ ا وﺻﻒ ﺮي . ﻨﻈ ﻟ ا م ﺪ ﻟ ا ﻢ ﻠ ﺔ : ﻋ ﻘ ﺑ ﺎ ﺎتﺳ ﺒ ﻠ ﺘﻄ ﻣ ﻤﻲ . ﻠ ﺪﺳ ﻤ ﺣ أ . أ ﺎق ﻤﺴ ﻟ ا رس ﺪ ﻣ د . ﻮ ﻷﺳ ا م ﺎ ﺘﺴ ﺑ ا ب . ﺎن . ﻘ ﺪﻟ ﻤ أ . ﻣﺤ ﺪ ﺎﻋ رسﻣﺴ ﺪ ﻣ ﺮي . ﻤﺼ ﻟ ا ة ﺪ ﯾ ﺎ ب . ﻋ م . ﺪ ﻟ ا ﺎت ﻧ ﻮ ﻜ ﻠﻰﻣ ﺮفﻋ ﻌ ﺘ ﻟ .١ ا ﺎق : ﻤﺴ ﻟ ا اف ﺪ ھ أ ﺔ . ﯿ ﺒ ﻟﻄ ا ﺎت ﻠﺤ ﻄ ﻤﺼ ﻟ ا ﻌﺾ ﻠﻰﺑ ﺮفﻋ ﻌ ﺘ ﻟ .٢ ا ء . ﺎ ﯿﻀ ﺒ ﻟ ا ءو ا ﺮ ﻤ ﺤ ﻟ ا م ﺪ ﻟ اتا ﺮ ﻜ ﻟ ﺔ ﯿ ﻌ ﯿ ﺒ ﺮﻃ ﯿ ﻐ ﻟ ا ل ﺎ ﻜ ﻷﺷ ا ﻌﺾ ﻠﻰﺑ ﺮفﻋ ﻌ ﺘ ﻟ .٣ ا ﺎ . ﮭ ﺋ ا ﺮ إﺟ ﺔ ﯿ ﻔ ﯿ ﻛ ﺎو ﮭ ﺑ ﺔ ﻘ ﻠ ﻌ ﺘ ﻤ ﻟ ا ﺎت ﻮﺻ ﺤ ﻔ ﻟ ا ﻠﻰ ﺮفﻋ ﻌ ﺘ ﻟ .٤ ا ﺔ . ﻔ ﻠ ﺘ ﻤﺨ ﻟ ا ﺎغ ﺒ ﻷﺻ ﻠﻰا ﺮفﻋ ﻌ ﺘ ﻟ .٥ ا ﯿﻦ . ﺑ ﻮ ﻠ ﻮﺟ ﻤ ﯿ ﮭ ﻟ ا ﻞ ﺮقﻓﺼ ﻠﻰﻃ ﺮفﻋ ﻌ ﺘ ﻟ .٦ ا ﺎ . ﯿ ﻤ ﯿ ﻧ اﻷ ﺔﻣﻦ ﻔ ﻠ ﺘ ﺨ ﻤ ﻟ ا اع ﻮ ﻧ ﻷ ﻠﻰا ﺮفﻋ ﻌ ﺘ ﻟ .٧ ا ﺔ ﯿ ﻤ ﯿ ﻠ ﻌ ﺘ ﻟ ا ﮫ ﺗ ﺮ ﯿ ﻞﻣﺴ ﻤ ﻜ ﯾ ﻜﻲ ﻟ ﯿﺢ ﻟﺼﺤ ا ﯾﻖ ﺮ ﻄ ﻟ ا رﻓﻲ ا ﺮ ﻤ ﺘ ﻻﺳ ا ﻟﺐ ﺎ ﻄ ﻟ ا ﻜﻦ ﻤ ﺎتﺗ ﻣ ﻠﻮ ﻌ ﻤ ﻟ ا رﻣﻦ ﺪ ﻠﻰﻗ ﻮلﻋ ﻟﺤﺼ ا أن ﻊ ﻗ ﻮ ﺘ ﻤ ﻟ ا ﺗﺞ ﺎ ﻨ ﻟ ا ﺔ ﻔ ﻠ ﺘ ﺨ اضﻣ ﺮ ﻣ أ ﻠﻰ ﺮفﻋ ﻌ ﺘ ﻟ ا ﻟﺐ : و ﺎ ﻟﻄ ا ﮫ ﯿ ﻠ ﻞﻋ ﯾﺤﺼ ر . ﻮ ﺣﻀ ﺔو ﯿ ﻠ ﻤ ﻌ ﺮﻣ ﯾ ر ﺎ ﻘ ﺎتﺗ رﺟ 10 د ﺎت : رﺟ ﺪ ﻟ ا ﻊ ﯾ ز ﻮ ﺗ ﺋﺢ . ا ﺮ ﺔﺷ ﯾ ؤ ﺔر رﺟ 30 د ﺋﻲ ﺎ ﮭ ﻠﻲﻧ ﻤ ﺎنﻋ ﺤ ﺘ ﻣ 10 ا ﺋﻲ ﺎ ﮭ ﺮيﻧ ﻈ ﺎنﻧ ﺤ ﺘ ﻣ 50 ا ﻔﻲ . ﺮيﻧﺼ ﻧﻈ ﺎن ﺘﺤ ﻣ ا ﺎت : ﻧ ﺎ ﺘﺤ ﻣ ﻻ ا ﯾﺦ ر ﺎ ﺗ ﻠﻲ . ﻤ ﺎنﻋ ﺘﺤ ﻣ ا ﺎ ﻘ ﺎتﻻﺣ ﻧ ﺎ ﺘﺤ ﻣ ﻻ ا د ﺪ ﺤ ﺗ ﺮي . ﻧﻈ ﺎن ﺘﺤ ﻣ ا ٢
Table of ContentsEXCERCISEBlood smearNormal cell maturation.Red blood cell morphology.Reticulocyte count.Detection of sickle cell.Chromatography (determination of HbA2, Hb F).G6PD.Sugar water screening testBlood sucrose testOsmotic fragility test.Automated hematology cell counters.Special stain.٣page41519263032364041424553
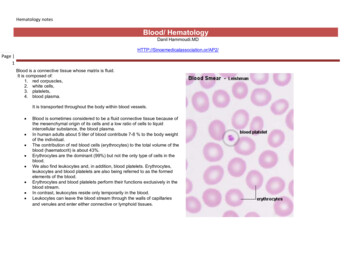
BLOOD SMEARPREPARATION AND STAININGI. Preparation of blood smear: Lab objective The student will prepare at least five slide smears which are even, smooth and have anacceptable feathered edge. The student will stain two of the above smears with Wright’s stain so that all formedelements are readily identifiable according to criteria outlined. SpecimenEDTA anticoagulated blood is preferred. Blood smears can also be made from finger stickblood directly onto a slide. Reagents, equipment. and supplies(a) SpreadersSelect a glass microscope slide with at least one smooth end.Each spreader can be used repeatedly provided that the spreading edge remains smooth. Theedge must be wiped carefully and dried before and after each use, and the slide must bediscarded if the spreading edge becomes chipped(b) Clean slidesIt is essential to use clean, dry, dust-free slides: remember that grease and residual detergentare equally liable to spoil a blood film.New slides: Boxes of clean grease-free slides may be available. If not, proceed as follows.Leave overnight in a detergent solution. Then wash thoroughly in running tap-water, rinsein distilled water if available and wipe dry with a clean linen cloth. Before use, wipe thesurface with methylated spirits (95% ethanol) or methanol and dry with a clean cloth; thenkeep covered to avoid having dust settle on the surface.Used slides: Discard in detergent solution, heat to about 60oC for 20 minutes. Then wash inrunning tap water, rinse in distilled water if available and treat as for new slides as describedabove.0 Procedure Three methods may be used to make blood smears:1. The cover glass smear2. The wedge smear3. The spun smear. The spun smear requires an automatic slide spinner. For the purpose of this labexercise, we will use the wedge smear.1. Fill a capillary tube three-quarter full with the anticoagulated specimen or a woodenstick.2. Place a drop of blood, about 2 mm in diameter approximately a inch from the frostedarea of the slide.3. Place the slide on a flat surface, and hold the narrow side of the non frosted edgebetween your left thumb and forefinger.4. With your right hand, place the smooth clean edge of a second (spreader) slide on thespecimen slide, just in front of the blood drop.5. Hold the spreader slide at a 30 angle, and draw it back against the drop of blood.٤
6. Allow the blood to spread almost to the edges of the slide.7. Push the spread forward with one light, smooth, and fluid motion. A thin film of bloodin the shape of a bullet with a feathered edge will remain on the slide.8. Label the frosted edge with patient name, ID# and date.9. Allow the blood film to air-dry completely before staining. (Do not blow to dry. Themoisture from your breath will cause RBC artifact.) Procedure notes1. A good blood film preparation will be thick at the drop end and thin at the opposite end.2. As soon as the drop of blood is placed on the glass slide, the smear should be madewithout delay. Any delay results in an abnormal distribution of the white blood cells,with many of the large white cells accumulating at the thin edge of the smear.3. The blood smear should occupy the central portion of the slide and should not touch theedges.4. The thickness of the spread when pulling the smear is determined by the1) angle of the spreader slide (the greater the angle, the thicker and shorter the smear).2) size of the blood drop .3) speed of spreading.5. If the hematocrit is increased, the angle of the spreader slide should be decreased.6. If the hematocrit is decreased, the angle of the spreader slide should be increased.٥
7. Common causes of a poor blood smear:a. Drop of blood too large or too small.b. Spreader slide pushed across the slide in a jerky manner.c. Failure to keep the entire edge of the spreader slide against the slide while making thesmear.d. Failure to keep the spreader slide at a 30 angle with the slide.e. Failure to push the spreader slide completely across the slide.f. Irregular spread with ridges and long tail: Edge of spreader dirty or chipped; dustyslideg. Holes in film: Slide contaminated with fat or greaseh. Cellular degenerative changes: delay in fixing, inadequate fixing time or methanolcontaminated with water8. Although this is the easiest and most popular methods for producing a blood smear, itdoes not produce a quality smear. The WBC's are unevenly distributed and RBCdistortion is seen at the edges. Smaller WBC's such as lymphocytes tend to reside in themiddle of the feathered edge.9. Large cells such as monocytes, immature cells and abnormal cells can be found in theouter limits of this area.10. Spun smears produce the most uniform distribution of blood cells.11. Biologic causes of a poor smeara. Cold agglutinin - RBCs will clump together. Warm the blood at 37 C for 5 minutes,and then remake the smear.b. Lipemia - holes will appear in the smear. There is nothing you can do to correct this.c. Rouleaux - RBC’s will form into stacks resembling coins. There is nothing you cando to correct this.II. Fixing the films To preserve the morphology of the cells, films must be fixed as soon as possible afterthey have dried. It is important to prevent contact with water before fixation iscomplete. Methyl alcohol (methanol) is the choice, although ethyl alcohol ("absolutealcohol") can be used. To prevent the alcohol from becoming contaminated byabsorbed water, it must be stored in a bottle with a tightly fitting stopper and not leftexposed to the atmosphere, especially in humid climates. Methylated spirit (95%ethanol) must not be used as it contains water. To fix the films, place them in a covered staining jar or tray containing the alcohol for2-3 minutes. In humid climates it might be necessary to replace the methanol 2-3 timesper day; the old portions can be used for storing clean slides.٦
III. Staining the film: Romanowsky staining Romanowsky stains are universally employed for staining blood films and are generallyvery satisfactory. There are a number of different combinations of these dyes, which vary, in theirstaining characteristics.1. May-Grunwald-Giemsa is a good method for routine work.2. Giemsa stain is thought to produce more delicate staining characteristics.3. Wright's stain is a simpler method.4. Leishman's is also a simple method, which is especially suitable when a stained bloodfilm is required urgently or the routine stain is not available (e.g. at night).5. Field's stain is a rapid stain used primarily on thin films for malarial parasites. PrincipleThe main components of a Romanowsky stain are:A cationic or basic dye (methylene blue or its oxidation products such as azure B),which binds to anionic sites and gives a blue-grey color to nucleic acids (DNAor RNA), nucleoproteins, granules of basophils and weakly to granules ofneutrophilsAn anionic or acidic dye such as eosin Y or eosin B, which binds to cationic siteson proteins and gives an orange-red color to hemoglobin and eosinophilgranules.Wright’s Stain Materials:Slides can be stained manually or on automatic slide stainers. For this lab, we will use themanual method.1. Commercial Wright’s stain2. Commercial buffer3. Deionized water4. 3 Coplin jars5. Clothes pin or forceps for holding the slide6. Paper towels Procedure1. Attach a clothes pin (or use forceps) to the thick edge of the blood smear.2. Place the slide in the Coplin jar with Wright’s stain. Allow to stand 5-10 seconds.3. Raise the slide out of the stain and allow the majority of the stain to run off the slide.4. Place the slide in the first jar containing deionized water. Allow to stand 10-20seconds.5. Remove the slide carefully and dip several times in the second jar containing deionizedwater to rinse off the excess stain.6. Wipe off excess fluid from the back of the slide. Place the slide upright on a papertowel with the feathered edge up and allow to air dry.7. When completely dry, examine the smear with the microscope as follows: Low power (10x) scan8. Determine the overall staining quality of the blood smear.a. Stain should not be too dark or too pale.b. There should be no stain precipitate present on smear.٧
c. RBCs should be appropriate color of reddish pink.d. Lymphocytes have dark purple nuclei with varying shades of blue cytoplasm.e. Neutrophils have dark purple nuclei with reddish, granular cytoplasm.f. Monocytes have a lighter purple nucleus with a gray-blue cytoplasm.g. Eosinophils have bright red/orange granules.h. Basophils have dark purple nuclei and granules.9. Determine if there is a good distribution of the cells on the smear.a. Scan the edges and center of the slide to be sure there are no clumps of RBCs,WBCs or platelets.b. Scan the edges for abnormal cells.c. High power (40 x) scan10. Find an optimal area for the detailed examination and enumerations of cells.a. The RBCs should not quite touch each other.b. There should be no area containing large amounts of broken cells orPrecipitated stain.c. The RBCs should have a graduated central pallor.d. Nuclei and cytoplasm of WBCs should be the proper color.e. Platelets should be clearly visible. Notes on the staining procedure:1. Whichever method is used, it is important to select dyes that are not contaminated withother dyes or metallic salts.2. Staining time must be specific for each lot of stains and so we must follow the kitprocedure.3. Bone marrow time staining must be increased.4. Staining characteristics of a correctly stained normal film: NucleiPurple Cytoplasm ErythrocytesDeep pink NeutrophilsOrange-pink LymphocytesBlue; some small lymphocytes deep blue MonocytesGrey-blue BasophilsBlue Granules NeutrophilsFine purple EosinophilsRed-orange BasophilsPurple-black MonocytesFine reddish (azurophil) PlateletsPurple5. Staining faults Too faint: Stain deposit:Staining time too shortExcessive washing after stainingStain solution left in uncovered jar or trayStain solution not filteredDirty slides٨
6. The phosphate buffer controls the PH of the stain. If the PH is too acid, those cells orcell parts taking up an acid dye stain will stain pinker and the acid components thatstain with the basic dye show very pale staining. If the stain –buffer mixture is alkaline,the red blood cells will appear grayish blue and the white cell nuclei will stain verydeeply purple. Therefore, to stain all cells and cell parts well, the PH of the phosphatebuffer is critical.7. The staining rack must be exactly level to guard against uneven staining of the smear.8. Insufficient washing of the smears when removing the stain and buffer mixture maycause stain precipitate on the dried smear.9. Excessive rinsing of the stained smear will cause the stain to fade.IV. MANUAL DIFFERENTIAL Lab objective1. To determine the relative number of each type of white cell present in the blood byperforming differential cell counts on five relatively normal blood smears and five setsof abnormal blood smears within a 15% accuracy of the instructor's values.2. To determine within one qualitative unit the red cell, white cell, and plateletmorphology of each of the above blood smears.3. To determine within 20% accuracy an estimate of the white cell counts and theplatelet counts of each of the above blood smears. PrincipleA stained smear is examined in order to determine the percentage of each type of leukocytepresent and assess the erythrocyte and platelet morphology. Increases in any of the normalleukocyte types or the presence of immature leukocytes or erythrocytes in peripheral bloodare important diagnostically in a wide variety of inflammatory disorders and leukemia.Erythrocyte abnormalities are clinically important in various anemias. Platelet sizeirregularities are suggestive of particular thrombocyte disorders. SpecimenPeripheral blood smear made from EDTA-anticoagulated blood. Smears should be madewithin 1 hour of blood collection from EDTA specimens stored at room temperature toavoid distortion of cell morphology. Unstained smears can be stored for indefinite periodsin a dry environment, but stained smears gradually fade unless coverslipped. Reagents, supplies and equipment Manual cell counter designed for differential counts Microscope, immersion oil and lens paper Procedure1. Focus the microscope on the 10X objective (low power). Scan the smear to check forcell distribution, clumping, and abnormal cells. In scanning the smear it is importantto note anything unusual or irregular, such as rouleaux or RBC clumping.٩
2. Examine the peripheral edge of the smear. If there is an increased number of white cellin this area, the differential count is inaccurate. Most of the cells at the edge of thesmear are the large white cells, namely neutrophils and monocytes. This, therefore,shows poor distribution of white cell types and the smear is unacceptable.3. If the smear is acceptable, estimate the white cell count by counting the number ofWBC in each of 5 or 6 low power fields. Average the numbers. Multiply the averageby 1000 and divide by 4. This number should be within 20% of the actual white cellcount. If it is not within this range, the white cell count and the estimation should berepeated.(Average # WBC per 5 fields) x 100/44. Using the 100 X oil objective, place a drop of oil on the slide and examine the smearfor platelets morphology and number. Find a thin area where red cells are notoverlapping. In the appropriate area, count the number of platelets on about 4 or 5successive fields.5. Average the number and multiply by 20,000 to obtain a rough estimate of the plateletcount. A field with a normal platelet count would contain 8-20 platelets on 100X.6. If platelets are clumped, this should be noted and one of the following comments addedto the report:a. Platelet count may not be accurate due to clumping. Platelets appear adequate.b. Platelet count may not be accurate due to clumping. Platelets appear decreased.c. Platelet count may not be accurate due to clumping. Platelets appear increased.d. Platelet count may not be accurate due to clumping. Platelets appear too clumpedfor accurate estimate.7. To perform the differential, choose the portion of the smear where there is closeproximity but little overlapping of the red cells. They should have a central pallor.You should use the 100 X objective or 50X if available.8. Begin the count in the thin area of the slide and gradually the slide as shown below.Count each white cell seen and record on a differential cell counter, until 100 whitecells have been counted. If any nucleated red cells (NRBCs) are seen during thedifferential count, enumerate them on a separate counter. They are not to be includedin the 100-cell differential count. They are reported as #NRBC/100 WBCs and theWBC count must be corrected if there are 10 NRBCs / 100 WBC. Reporting results Results are expressed as a percentage of the total leukocytes counted. It is also helpful to know the actual number of each white cell type per µL ofblood. This is referred to as the absolute count and is calculated as follows:Absolute number of cells/µl % of cell type in differential x white cell count Reference values vary depending on age. For this exercise, the following valueswill be usedCell TypeTotal WBC x 103/µLNeutrophils %Lymphocyte %Monocyte %Eosinophil %Basophil %Birth10-261 mo5-19.56 yr4.3-13.514 -145-5535-450-81-40-150-6530-400-100-40-1١٠
White blood cells White blood cells, or leukocytes, are classified into two main groups; granulocytesand nongranulocytes (also known as agranulocytes).1. The granulocytes, which include neutrophils, eosinophils, and basophils, havegranules in their cell cytoplasm. Neutrophils, eosinophils, and basophils alsohave a multilobed nucleus. As a result they are also called polymorphonuclearleukocytes or "polys." The nuclei of neutrophils also appear to be segmented, sothey may also be called segmented neutrophils or "segs."2. The nongranulocytes white blood cells, lymphocytes and monocytes, do nothave granules and have nonlobular nuclei. They are sometimes referred to asmononuclear leukocytes. Leukocytosis, a WBC above 10,000, is usually due to an increase in one of the five types ofwhite blood cells and is given the name of the cell that shows the primary increase. Neutrophilic leukocytosis neutrophilia Lymphocytic leukocytosis lymphocytosis Eosinophilic leukocytosis eosinophilia Monocytic leukocytosis monocytosis Basophilic leukocytosis basophilia1. Neutrophils Neutrophils are so named because they are not well stained by either eosin, a redacidic stain, nor by methylene blue, a basic or alkaline stain. Neutrophils are also known as "segs", "PMNs" or "polys" (polymorphonuclear). They are the body's primary defense against bacterial infection. Normally, most ofthe neutrophils circulating in the bloodstream are in a mature form, with the nucleusof the cell being divided or segmented. Because of the segmented appearance of thenucleus, neutrophils are sometimes referred to as "segs." The nucleus of less mature neutrophils is not segmented, but has a band or rod-likeshape. Less mature neutrophils - those that have recently been released from thebone marrow into the bloodstream - are known as "bands" or "stabs". Increased neutrophils count (neutrophilia) An increased need for neutrophils, as with an acute bacterial infection, will cause anincrease in both the total number of mature neutrophils and the less mature bands orstabs to respond to the infection. The term "shift to the left" is often used whendetermining if a patient has an inflammatory process such as acute appendicitis orcholecystitis. In addition to bacterial infections, neutrophil counts are increased in:١١
1.2.3.4.5.Many inflammatory processes.During physical stressWith tissue necrosis that might occur after a severe burnMyocardial infarction.Granulocytic leukemia. Shift to left Increased bands Means acute infection, usually bacterial.Shift to right Increased hypersegmented neutrophile. Decreased neutrophil count (neutropenia)This take place in the following: Typhoid fever Brucelosis Viral diseases, including hepatitis, influenza, rubella, and mumps. An great infection can also deplete the bone marrow of neutrophils andproduce neutropenia. Many drugs used to treat cancer produce bone marrow depression and cansignificantly lower the neutrophil count.2. EosinophilsEosinophils are associated with antigen-antibody reactions.1. The most common reasons for an increase in the eosinophil count are Allergicreactions such as hay fever, asthma, or drug hypersensitivity.2. Parasitic infection3. Eosinophilic leukemiaDecreases in the eosinophil count may be seen when a patient is receiving corticosteroid drugs.3. Basophils The purpose of basophils is not completely understood. Basophils are phagocytes and contain heparin, histamines, and serotonin. Tissue basophils are also called" mast cells." Similar to blood basophils, theyproduce and store heparin, histamine, and serotonin. Basophile counts are used to analyze allergic reactions. An alteration in bone marrow function such as leukemia or Hodgkin's diseasemay cause an increase in basophils. Corticosteroid drugs, allergic reactions, and acute infections may cause thebody's small basophile numbers to decrease.4. Lymphocytes Lymphocytes are the primary components of the body's immune system. They arethe source of serum immunoglobulins and of cellular immune response. As a result,they play an important role in immunologic reactions. All lymphocytes are produced in the bone marrow. The B-cell lymphocyte alsomatures in the bone marrow and controls the antigen-antibody response that isspecific to an offending antigen; the T-cell lymphocyte matures in the thymusgland, the T cells are the master immune cells of the body, consisting of T-4 helpercells, killer cells, cytotoxic cells, and suppressor T-8 cells. In adults, lymphocytes are the second most common WBC type after neutrophils,hence lymphocytosis is usually associated with neutropenia and lymphopenia is١٢
associated with neutropeina. In young children under age 8, lymphocytes are morecommon than neutrophils. Lymphocytes increase (lymphocytosis) in: Many viral infections Tuberculosis. Typhoid fever Lymphocytic leukemia.A decreased lymphocyte (lymphopenia) count of less than 500 places a patient at very high riskof infection, particularly viral infections.5. Monocytes Monocytes are the largest cells in normal blood. They act as phagocytes in someinflammatory diseases and are the body's second line of defense against infection. Diseases that cause a monocytosis include: Tuberculosis Brucellosis Malaria Rocky Mountain spotted fever. Monocytic leukemia Chronic ulcerative colitis Procedure notes1. A well-made and well-stained smear is essential to the accuracy of the differentialcount. The knowledge and ability of the cell morphologist is critical to high-qualityresults.2. Before reporting significant abnormalities such as blasts, malaria or other significantfinding on a patient’s differential, ask a more experienced tech to review the smear forconfirmation. In clinical settings where a pathologist or hematologist is present, thesmear is set aside for Pathologist Review.3. If disrupted cells are present such as smudge cells or basket cells, not them on thereport. It may be necessary to make an albumin smear to prevent the disruption of thecells. RBC morphology and WBC morphology must always be performed on the nonalbumin smear.4. When the WBC is very low (below 1,000/µL), it is difficult to find enough WBCs toperform a 100-cell differential. In this situation, a differential is usually performed bycounting 50 cells. A notation on the report must be made that only 50 white cells werecounted. Multiply each percentage x 2.5. When the WBC is very high ( 50,000/µL), a 200-cell diff may be performed toincrease the accuracy of the diff. The results are then divided by 2 and a note made onthe report that 200 white cells were counted.6. Never hesitate to ask questions concerning morphology or the identification of cells.The differential is one of the most difficult laboratory tests to learn. In fact, learningabout cells and their morphology is a process that continues for as long as you performdifferentials.١٣
7. It is permissible to use a 50x objective to perform a differential, however keep thefollowing points in mind:1) If the WBC is increased, you should use the 100x to ensure that you will notskip cells in a field.2) If you are having trouble identifying a cell, you must switch to the 100x in orderto get a more detailed view.Characteristics of blood cellsErythrocyte: Usually biconcave and circular outline, devoid of a nucleus. Number in manvaries between 5 and 5.5 million per cubic mm of blood. Erythrocytes carry oxygen from thelungs to the tissues and carbon dioxide from the tissues to the lungs.Neutrophil: Compare sizes of the neutrophil and the erythrocyte. Lobulated nucleus,individual lobes connected by thin bridges. Cell type-specific cytoplasmic granules are small.Neutrophils constitute 40 to 75 per cent of the total white blood cell count. The number ofneutrophils increases in inflammation, and they act as the first line of defense against invadingpyogenic organisms.Eosinophil: Nucleus bilobed. Cell type-specific cytoplasmic granules are large and uniform insize and stain intensely red with acid dyes. They constitute 1 to 3 per cent of total white countand increase in number in allergic states and in parasitic infections.Basophil: The nucleus is large but less lobulated than other white blood cells. Cell typespecific cytoplasmic granules are large and variable in size and have a strong affinity for basicdyes. They constitute 0.5 to 1 per cent of white count and are believed to synthesize the heparinand histamine found in circulating blood.Normal Cell Maturation١٤
Introduction- Red blood cells are the most common type of blood cell and the vertebrate body's principalmeans of delivering oxygen from the lungs or gills to body tissues via the blood.- Red blood cells are also known as RBCs, haematids or erythrocytes (from Greek erythros for"red" and kytos for "hollow", with cyte nowadays translated as "cell"). "RBCs" should infact be referred to as "corpuscles" rather than "cells". Indeed, a 'cell' contains a nuclearelement, mature RBC's do not contain a nucleus in mammals.- Mammalian erythrocytes are biconcave disks: flattened and depressed in the center, with adumbbell-shaped cross section. This shape (as well as the loss of organelles and nucleus)optimizes the cell for the exchange of oxygen with its surroundings.- Erythrocytes in mammals are a nucleate when mature; meaning that they lack a cell nucleusand thus have no DNA, also lose their other organelles.- As a result of the lack of nucleus and organelles, the cells cannot produce new structural orrepair proteins or enzymes and their lifespan is limited.- Erythrocytes consist mainly of hemoglobin, and the color of erythrocytes is due to the hemegroup of hemoglobin.- The diameter of a typical human erythrocyte disk is 6–8 µm, much smaller than most otherhuman cells.- A typical erythrocyte contains about 270 million hemoglobin molecules, with each carryingfour heme groups.- Adult humans have roughly 2–3 1013 red blood cells at any given time (women have about4 to 5 million erythrocytes per microliter (cubic millimeter) of blood and men about 5 to 6million; people living at high altitudes with low oxygen tension will have more). Red bloodcells are thus much more common than the other blood particles.Blood cells go through several stages of development; progression from one stage to the next isnot abrupt, so frequently the cell being studied may be between stages (when this occurs thecell is generally given the name of the mature stage). As a cell transforms from the primitiveblast stage to the mature form found in the blood, there are changes in the cytoplasm, nucleus,and cell size. Normally, all three of these changes occurs gradually and simultaneously. Insome disease states, however, changes will take place at different rates. For example, thecytoplasm may mature more quickly than the nucleus. This occurrence is termedasynchronism.Cytoplasmic MaturationThe immature cytoplasm usually stain a deep blue color (basophilic) because of the highcontent of RNA present as the cell matures, there is a gradual loss of cytoplasmic RNA andtherefore a lessening of blue color. In some cells (for example, the myelogenous cells),١٥
granules appear in the cytoplasm as the cell matures. At first, these granules are few andnonspecific. As the cell matures further, these granules increase in number and take onspecific characteristics and functions. The amount of cytoplasm in relationship to the rest of thecell usually increases as the cell matures.Nuclear MaturationThe nucleus of the immature cell is round oval and is large in proportion to the rest of the cell.As the cell matures the nucleus decreases in relative size and may or may not take on variousshapes (depending on the cell type). The nuclear chromatin transforms from a fine, delicatepattern to become more coarse and clumped on the mature form, and the staining propertieschange from a reddish purple to a bluish purple. Nucleoli present in early stages of ce
The spun smear requires an automatic slide spinner. For the purpose of this lab exercise, we will use the wedge smear. 1. Fill a capillary tube three-quarter full with the anticoagulated specimen or a wooden stick. 2. Place a drop of blood, about 2 mm in diameter ap