Transcription
Washington State Department of Health (DOH)Latent Tuberculosis InfectionA Quick Guide to Case ManagementDOH TB Program2014DOH 343-122 October 2014
Table of ContentsIntroduction. 2Diagnosing Latent TB Infection Flow Chart . 3Section One: Diagnosing TB Infection . 4How to Interpret a Tuberculin Skin Test Reaction. 5Labs Available to Perform IGRA Testing . 7TB Testing Risk Assessment Form . 9Tuberculosis Laboratory Diagnostics Summary . 11Section Two: Initiating Treatment . 12Recommended Drug Regimens for Treatment of LTBI . 13Consent for LTBI Treatment . 16Section Three: Case Management . 18LTBI Case Management: Monthly Patient Assessment . 19-20Section Four: Dispositioning the Patient . 22Tuberculosis Treatment Summary . 23Section Five: Additional Resources . 24
IntroductionThis guide is intended for providers who care for individuals who have or may be at risk for latenttuberculosis infection (LTBI). LTBI is the presence of Mycobacterium tuberculosis in the body withoutsigns and symptoms, or radiographic or bacteriologic evidence of tuberculosis (TB) disease.In the United States, an estimated 9-14 million people have LTBI. Without treatment, approximately 510% of persons with LTBI will progress to TB disease at some point in their lifetime unless LTBI therapy isinitiated. Identifying and treating those at highest risk for TB disease will help move toward eliminationof the disease.This document is not meant to be used as a substitute for the comprehensive guidelines published bythe Centers for Disease control and Prevention (CDC) and by Washington State Department of Health(DOH), but rather as a ready and useful reference that highlights the main points of those guidelines.In this document you will find summarization of the main topics related to LTBI diagnosis and casemanagement, links to useful tools and resources, as well as sample forms that can be modified for useby your facility.2
Diagnosing Latent TB InfectionPerform TB test(TST or IGRA)TB test PositiveTB test NegativeObtain chestradiographRadiograph Abnormal*Obtain laboratorydiagnostics (sputums)**Sputums Positivefor TB disease***Treat for activeTB diseaseSputums Negativefor TB diseaseRadiograph NormalTreat for LTBI*Consider notifying/consulting withlocal health department.** Collect 3 sputums for AFB smear,culture, and nucleic acid amplificationtesting (NAAT).*** Notify the local health departmentand consult and/or refer patient tothem for treatment.Treat for LTBI3
Section One: Diagnosing TB InfectionTests for TB InfectionTuberculin Skin Test (TST)The tuberculin skin test is administered intradermally using the Mantoux technique by injecting 0.1 mlof 5 TU purified protein derivative (PPD) solution. If a person is infected, a delayed-type hypersensitivityreaction is detectable 2-8 weeks after infection. The reading and interpretation of TST reactions shouldbe conducted within 48 to 72 hours of administration by a trained health care professional. iOnline training on administration of the TST using the Mantoux method is available at:http://www2c.cdc.gov/podcasts/player.asp?f 3739Key Points Almost everyone can receive a TST, including infants, children, pregnant women, people livingwith HIV, and people who have had a BCG vaccination. People who had a severe reaction to aprecious TST should not receive another TST.iiThe TST should not be performed on a person who has written documentation of either aprevious positive TST result or treatment for TB disease.i Once positive, a TST will likely alwaysreact positive on subsequent testing.Interpretation of the TST result is the same for persons who have had BCG vaccination.iA positive TB test indicates that a person has been infected with TB, but does not differentiatebetween latent and active TB.ii4
How to Interpret a Tuberculin Skin Test ReactionInduration Size5 mm or moreConsidered Positive In: HIV-infected personsRecent contacts of a person with infectious TB diseasePersons with fibrotic changes on chest radiograph consistentwith prior TBOrgan transplant recipientsPersons who are immunocompromised for other reasons (e.g.,taking equivalent of 15 mg/day of prednisone for 1 month ormore or those taking TNF-alpha antagonisits)10 mm or more 15 mm or more Foreign-born persons from countries with a high TB incidenceor prevalence (e.g., most countries in Africa, Asia, LatinAmerica, Eastern Europe, the former USSR, or from refugeecamps)Injection drug user’sResidents and employees in high-risk, congregate settings (e.g.,correctional institutions; long-term residential care facilities,such as nursing homes, mental institutions, etc.; hospitals andother healthcare facilities; homeless shelters; and refugeecamps)Mycobacteriology laboratory personnelPersons with other medical conditions that increase the risk ofTB disease (e.g., diabetes, chronic renal failure or onhemodialysis, head and neck cancer)Children younger than 4 years of age, or children andadolescents exposed to adults in at high risk for TB diseasePersons with no known risk factors for TB5
BCG VaccineThe BCG vaccine is currently used in many parts of the world where TB is common to protect infants andyoung children from serious, life-threatening disease. BCG vaccination is not recommended in the U.S.The question of the effect of BCG vaccine on TST results often causes confusion. TST reactivity caused byBCG vaccine generally wanes with the passage of time, but periodic skin testing may prolong (boost)reactivity in vaccinated persons. A history of BCG vaccination is not a contraindication for tuberculin skintesting or treatment for LTBI in persons with positive TST results. TST reactions should be interpretedregardless of BCG vaccination history.iInterferon-Gamma Release Assays (IGRAs) use M.tuberculosis specific antigens that do not cross reactwith BCG and therefore, do not cause false positive reactions in BCG recipients.iInterferon –Gamma Release Assays (IGRAs)Like the TST, IGRAs are used to determine if a person is infected with M. tuberculosis. TheQuantiFERON - TB Gold In-Tube test (QFT-GIT), and T-SPOT. - TB are the two available IGRA tests. Theadvantages of IGRAs include that they are unaffected by BCG and most environmental mycobacteria,and that a positive and negative control is built into the test which minimizes false positive and negativeresults.iFor more information on QFT-GIT and T-SPOT see: www.quantiferon.com and www.tspot.comKey Points Blood samples must be processed within 8-16 hours. Blood samples must be collected using specific tubes and collection technique. Limited data exist on use in children younger than 5 years of age. IGRAs do not cross react with BCG vaccine.i Once positive, an IGRA will likely always react positive on subsequent testing.6
Labs Available to Perform IGRA TestingEvergreen Hospitalth12040 NE 128 StKirkland, WA 98034Ph: 425-899-3900Fax: 425-899-3901Group HealthLocations throughout Washington.Click on link to find the nearestmedical center.LabCorp-Northwest RegionLocations throughout Washington.Click on link to find the nearestlaboratory.Overlake Hospital Medical Centerth1135 116 Ave NE Ste 170Bellevue, WA 98004425-688-5106Paclab Network LaboratoriesClick on link to find the nearestlaboratory.425-688-9274PAML – Pathology AssociatesMedical Laboratories110 W Cliff AveSpokane, WA 99204PAML Client Services (statewide):800-541-7891Bellevue/Seattle: 888-472-2522Olympia: 888-910-6156Fax: 509-924-0002Courier Services: 800-541-7891Providence Everett916 Pacific AvenueEverett, WA 98201425-261-2000Providence St. Peter HospitalClinical Laboratory413 Lilly Road NEOlympia, WA 98506360-493-5181Public Health – Seattle and King County325 Ninth AveSeattle, WA 98104Ph: 206-744-8950Fax: 206-731-8963Quest Diagnostics1737 Airport Way S, Suite 200Seattle, WA1-866-697-8378Seattle Children’s4800 Sand Point Way NESeattle, WA 98105866-987-2000 (Toll Free)Tacoma General Hospital(Laboratories Northwest)thth1003 South 5 – 4 FloorTacoma, WA 98405253-403-1187Tri-Cities Laboratory7131 West Grandridge Blvd.Kennewick, WA 99336509-736-0100UW Medical Center1959 NE Pacific StSeattle, WA 98195206-598-6131UW MC Fax 206-598-7937Harborview Fax: 206-744-48507
Selecting a Test to Detect TB Infection IGRAs are the preferred method of testing for:--Groups of people who have poor rates of returning to have the TST read--Persons who have received BCG vaccine TST is the preferred method for testing for:--Children under the age of 5 years Either TST or IGRA may be used without preference for other groups that are tested for LTBI.iFor more information on selecting a test for TB infection please see:http://www.cdc.gov/mmwr/pdf/rr/rr5905.pdfKey Point Routine testing with both TST and IGRAs is NOT recommended.iAt the time of testing the person should be evaluated for risk of TB infection and disease, symptoms ofTB disease, and any TB history such as prior positive TB tests and completion of TB therapy. A thoroughrisk assessment will help in choosing a testing method, interpreting TB test results, and provide usefulinformation regarding potential treatment options.The following form is an example TB risk assessment form:8
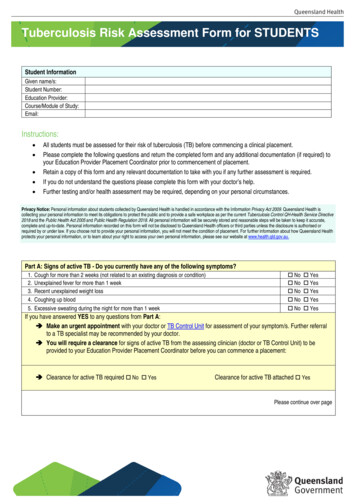
TB Testing Risk Assessment ale:Phone #:City:State:Zip:TB HistoryDocumentation of Prior TB Test: YesNoDate:Documentation of Prior TB Treatment Completion: YesResult:NoSymptomsNoneCoughHemoptysis (blood in sputum)FeverNight SweatsUnusual FatigueWeight LossAnorexia (loss of appetite)Dyspnea (shortness of breath)Chest PainHoarsenessIf yes to any of the above, please specify for how long:Risk AssessmentMedical Risk:HIV TSTAbnormal CXRIV drug use/substance abuseDiabetesSteroid/immunosuppressive medicationChronic Renal FailureCancer/LeukemiaPulmonary ScilicosisIntestinal Bypass SurgeryAge 5yrsTB ExposurePopulation Risk: (Live or work in)Homeless ShelterPrison/JailHealthcare FacilityNursing HomeForeign BornTB TestingCurrent Medications:TST Date:Time:Recent Vaccinations: YesRead Date:Result (mm):Positive:NoNegative:PPD Solution Lot #:ConsentI consent to a TB test for tuberculosis for myself.ORI consent to a TB test for tuberculosis for my child,Signature:.Date:9
Follow-up for Positive TB TestChest RadiographAll persons with a positive TB test should receive a chest radiograph. Chest radiographs helpdifferentiate between LTBI and pulmonary TB disease.iKey Points Persons 5 years of age should have a posterior-anterior view radiograph. Children under 5 years of age should have both posterior-anterior and lateral views. Periodic follow-up radiographs are not indicated regardless of whether treatment is completedexcept in unusual circumstances (e.g, contacts to patients with drug resistant TB).iRadiographic findings suggestive of active TB include: Air-space opacity or consolidation, often referred to as air-space disease Interstitial opacity Nodules or masses Thoracic lymphadenopathy Pulmonary cysts or cavities Pleural space abnormalitiesFor more information on TB Chest Radiology duct details.cfm?productID ONL-15Sputum ExaminationSputum examination is indicated for persons with positive TB test results and either an abnormal chestradiograph or the presence of respiratory symptoms (even when the chest radiograph is normal).iKey Points Three consecutive sputums should be collected 8-24 hours apart with one being and earlymorning sputum. Specimens should be refrigerated until sent to the laboratory. Order an Acid Fast Bacilli (AFB) smear and culture on each specimen. Nucleic Acid Amplification testing (NAAT) may be ordered through the Washington State PublicHealth Lab (WAPHL). Contact WAPHL at 206-418-5473 for ordering information.10
Tuberculosis Laboratory Diagnostics SummaryAFB SmearAFB CultureSpeciesIdentification Nucleic AcidAmplification Test(NAAT) Drug SensitivityTesting Drug ResistanceMutationDetection Genotyping Tests for the presence of any mycobacteriumResults available within 24 hoursProvides clue to potential infectivityDoes not differentiate between live and dead mycobacteriumPerformed in most laboratoriesGold standard for diagnosing TBResults typically available in 2-8 weeksOnly detects live mycobacteriumPerformed at WAPHL, Harborview, SeaKing PHL, PAML, UW, andcommercial labsPerformed automatically on positive cultures to determine the type ofmycobacterium present (ex. M.tb, M. avium, M. gordonae)One of the following methods is used to identify the species:o DNA Probe (AccuProbe)o Hsp65 sequencingo High Performance (or Pressure) Liquid Chromatography (HPLC)Detects TB DNAPerformed after AFB smear, if ordered (more sensitive on smear positivespecimens)A positive NAAT is considered a confirmed case of TBA negative NAAT does not rule out TBResults available in 24-72 hoursDoes not differentiate between live and dead mycobacteriumTwo methods for NAA testing include:o Polymerase Chain Reaction (PCR) performed at WAPHLo Hsp65 Sequencing performed at UWFirst-line (SIRE and usually PZA) performed automatically, using MGITinstrument, on culture positive specimensAvailable within 30 days of culture positive resultPerformed at Harborview, PAML, or WAPHLSecond-line performed at WAPHL or CDC using plate or Agar ProportionMethod, if first-line resistance detectedDetects common mutations located within specific regions of TB DNAPerformed when requested on NAAT or culture positive specimensTwo methods for detecting mutations include:o Drug Resistance Screening by Sequencing (DRSS) performed atWAPHLo Molecular Detection of Drug Resistance (MDDR) performed at CDCDetected mutation does not always mean total resistance to the drug(s)Performed automatically on culture positive specimensDetermines the strain of TB and whether it matches other strains of TBPerformed by a CDC contracted lab in MichiganAcronyms: Washington State Public Health Lab (WAPHL), Seattle and King County Public Health Lab (SeaKing PHL),Pathology Associates Medical Laboratory (PAML), Univiersity of Washington (UW), Centers for Disease Control andPrevention (CDC), Streptomycin, Isoniazid, Rifampin, Ethambutol (SIRE), Pyrazinamide (PZA)11
Section Two: Initiating TreatmentDecision to TreatThe decision to initiate or forego treatment for LTBI should be made by weighing a person’s risk forprogression to active TB disease, risk for potentially harmful side effects from the medication, andlikelihood of patient adherence. The following tool may help you estimate the risk of active TB forpersons with a TST reaction 5mm and/or a positive IGRA: http://tstin3d.com/en/calc.htmlKey Points There is no age cutoff for LTBI treatment Never begin treatment for LTBI until active TB disease is ruled outChoosing a LTBI Treatment RegimenEach LTBI treatment regimen differs regarding risk for side effects, drug-drug interactions, and length oftreatment. With this in mind, an appropriate regimen should be chosen after considering a person’shealth status, other medications prescribed, and life circumstances.The following printable pocket guide describes the different LTBI Treatment Regimens:12
Tuberculosis ServicesPhone: (360) 236-3443Fax: (360) 236-3405Email: TBServices@doh.wa.govRECOMMENDED DRUG REGIMENS FOR TREATMENT OF LTBIDrugINH*RIFInterval/DurationDaily for 9mos.Twiceweekly for9 mos.Daily for 4mos.Daily for atleast 6mos.Onceweekly for12 wks.Adult, 15yrs,Dosage (max)5 mg/kg (300mg)Pediatric, 15yrs,Dosage (max)10-20 mg/kg (300mg)15mg/kg (900mg)20-30 mg/kg10 mg/kg(600mg)10-20 mg/kg (600mg)Criteria forCompletion270 doseswithin 12 mos.76 doses within12 mos.120 doseswithin 6 mos.180 doseswithin 9 mos.DAILY INH PREFERRED FOR ALL PERSONSNot indicated for persons exposed to INH-resistant TB DOT must be used with twice-weekly dosingFor contacts of patients with INH-resistant, RIF-susceptible TB or whenshorter course treatment is preferredIn HIV-infected persons, protease inhibitors or NNRTIs should not beadministered concurrently with RIF DOT must be used with this regimen11 or 12 dosesOnly for 12yrRifapentine:within 16 wks.10-14kg 300mg 14.1Do not use in children 2, or for HIV-infected patients receiving ARV25kg 450mgtherapy, or for pregnant women, or for persons exposed to INH or RIF25.1-32kg 600mgresistant TB32.1-49.9kg 750mg(900mg)INH is preferred for children 2-11, however, this regimen mayINH:considered when completion of 9 months of INH is unlikely and the risk15mg/kg (900mg)of TB disease is greatAbbreviations: INH Isoniazid, RIF Rifampin, RPT rifapentine, NNRTIs non-nucleoside reverse transcriptase inhibitors, ARV antiretroviral, DOT directly observedtherapy, mos. months, wks. weeks*Isoniazid for six months can be considered a complete course of LTBI therapy; however, it is not indicated for persons with HIV infection or fibrotic lesions, or forchildren, or for persons exposed to INH-resistant TBINH/RPTRifapentine:10-14kg 300mg 14.125kg 450mg25.1-32kg 600mg32.1-49.9kg 750mg(900mg)INH:15mg/kg (900mg)CommentsDrug InformationDrugINHAdverse Reactions Hepatitis Peripheral neuropathy Hypersensitivity reactionsOther reactions, including optic neuritis, athralgias,CNS changes, drug-induced lupus, headache,diarrhea, and cramping.Note: Pyridoxine (B6) supplements are alsorecommended for pregnant and breast feedingwomen, breast-feeding infants, children andadolescents on milk- and meat-deficient diets,children who experience parasthesias while takingINH, and those with HIV infectionMonitoringClinical monitoring isessential. Liver functionmonitoring isappropriate if givenwith other hepatotoxicmedications or if thereare symptoms ofhepatotoxicity. Monitorconcentrations ofphenytoin orcarbamazepine. Patient InstructionsMay take with foodAvoid alcoholAvoid antacid within one hour of takingINHCall doctor right away if:Loss of appetite for a few daysTiredness, weaknessStomach pain, nausea, or vomitingNumbness or tingling in fingers or toesBlurred vision, eye painYellow skin or eyes or dark-colored urineRifamycin allergy;Liver function Best taken without fooddue do drugmonitoring is Avoid wearing soft contact lensesinteractions, may beappropriate if given RIF decreases effectiveness of oralcontraindicated withwith other hepatotoxichormone-based birth control methodsconcurrent use ofmedications or if there Call doctor right away if:certain drugs (ARVs,are symptoms ofUnusual tiredness or loss of appetiteanticoagulants,hepatotoxicity; monitorSevere abdominal upsetmethadone, oraldrug concentrations ofFever or chillsh
Overlake 1Hospital Medical Center 1135 116th Ave NE Ste 170 Bellevue, WA 98004 425-688-5106 Paclab Network Laboratories Click on link to find the nearest laboratory. 425-688-9274 100 PAML – Pathology Associates Medical Laboratories 110 W Cliff Ave Spokane,