Transcription
5Radixin Knockdown by RNA Interference Suppresses Human Glioblastoma Cell Growth in Vitro and in VivoRESEARCH ARTICLERadixin Knockdown by RNA Interference Suppresses HumanGlioblastoma Cell Growth in Vitro and in VivoJun-Jie Qin1, Jun-Mei Wang1, Jiang Du1, Chun Zeng2, Wu Han2, Zhi-Dong Li2,Jian Xie2*, Gui-Lin Li1*AbstractRadixin, a member of the ERM (ezrin–radixin–moesin) family, plays important roles in cell motility, invasionand tumor progression. It is expressed in a variety of normal and neoplastic cells, including many types ofepithelial and lymphoid examples. However, its function in glioblastomas remains elusive. Thus, in this study,radixin gene expression was first examined in the glioblastoma cells, then suppressed with a lentivirus-mediatedshort-hairpin RNA (shRNA) method.We found that there were high levels of radixin expression in glioblastomaU251cells. Radixin shRNA caused down-regulation of radixin gene expression and when radixin-silenced cellswere implanted into nude mice, tumor growth was significantly inhibited as compared to blank control cells or nonsense shRNA cells. In addition, microvessel density in the tumors was significantly reduced. Thrombospondin-1(TSP-1) and E-cadherin were up-regulated in radixin- suppressed glioblastoma U251 cells. In contrast, MMP9was down-regulated. Taken together, our findings suggest that radixin is involved in GBM cell migration andinvasion, and implicate TSP-1, E-cadherin and MMP9 as metastasis-inducing factors.Keywords: Malignant glioblastoma multiform - small interference RNA - radixin - thrombospondin-1Asian Pac J Cancer Prev, 15 (22), 9805-9812IntroductionGlioblastoma (GBM) is a WHO grade IV tumor arisingfrom the astrocytes and represents the most common typeof primary central nervous system (CNS) malignanciesproviding for well over one half of all gliomas (Hoffmanet al., 2006; Schneider et al., 2010). Current treatmentmethods include surgical resection, radio-surgical,external irradiation, chemotherapy (Cheng et al., 2005;Stupp et al., 2006; Wen et al., 2006), and biologicaltherapy (Kjaergaard et al., 2005). Despite recent advancesin diagnostics and treatments, prognosis for patientswith this disease remains poor (Ng et al., 2007). One ofthe important reasons is that active cell migration andinvasion of GBM cells ultimately lead to ubiquitous tumorrecurrence and patient death (Xia et al., 2009). Althoughour understanding of GBM carcinogenesis has steadilyimproved, the factors that mediate GBM invasion are stillpoorly understood. Various studies investigate the genesand gene products involved in GBM cell migration andinvasion, like matrix metalloproteinase genes, growthfactors, cytokines, integrins, and cadherins (Nakada etal., 2007).Radixin, encoded by chromosome 11 (11 exons), isa member of the ERM (ezrin-radixin-moesin) family.It functions as a membrane-cytoskeletal crosslinker inactin-rich cell surface structures and is thereby thoughtto be essential for cortical cytoskeleton organization,cell motility, adhesion, and proliferation (Hoeflich andIkura, 2004). ERM proteins display a similar structuralorganization. They share extensive homology in theiramino-terminal domain, which is called the four-pointone, ezrin, radixin, moesin (FERM) domain (Gautreauet al., 2002). Spanning these globular domains is theα-helix-rich domain, termed the α-domain. Recentbiophysical studies indicate that the radixin α-domainis an extremely long linear monomer with an enhancednumber of electrostatic salt bridge interactions predicted tocontribute synergistically to its thermal stability (Hoeflichet al., 2003). Activated radixin has been shown to join actinfilaments to CD43, CD44, and ICAM1-3 cell adhesionmolecules and various membrane channels and receptors,such as the Na /H exchanger-3 (NHE3), cystic fibrosistransmembrane conductance regulator (CFTR), and theβ2-adrenergic receptor(Tsukita and Yonemura, 1999;Bretscher et al., 2002).Radixin is expressed in a variety of normal andneoplastic cells, including many epithelial and lymphoidcell types (Ramoni et al., 2002; Suda et al., 2011). Inpancreatic carcinomas, high radixin expression levelsDepartment of Neuropathology, Beijing Neurosurgical Institute, 2Department of Neurosurgery, Beijing Tiantan Hospital, CapitalMedical University; China National Clinical Research Center for Neurological Diseases; Center of Brain Tumor, Beijing Institutefor Brain Disorders, Beijing Key Laboratory of Brain Tumors, Beijing, China *For correspondence: liguilin2014@126.com,xiejianw@sina.com1Asian Pacific Journal of Cancer Prevention, Vol 15, 20149805
Jun-Jie Qin et alare associated with high metastatic, cell proliferation,survival, adhesion, and invasive potential (Chen et al.,2012). Ezrin was expressed in astrocytes in the centralnervous system (CNS). Increased ezrin expression isassociated with enhanced cell growth and poor malignanttumor prognosis, including gliomas (Geiger et al., 2000;Khanna et al., 2004; Tynninen et al., 2004). The observedeffects of ezrin overexpression and silencing on thecell malignant transformation indicate a role for ezrinin regulating tumor metastasis and progression (Curtoand McClatchey, 2004). However, the mechanisms ofradixin-mediated tumor development still require furtherelucidation. In this study, we investigated radixin’s effecton the growth and invasion ability of the glioblastomacell line U251.Experimental proceduresCells and antibodiesThe glioblastoma cell line U251 and normal astrocyteglial cells were purchased from the Saier Biotechnology(Tianjin, China), cultured in DMEM (GIBCO, GrandIsland, NY) supplemented with 10% fetal calf serum (FCS)and 1% L-glutamine (Invitrogen, Karlsruhe, Germany),and maintained at 37 C in 5% CO2. Rabbit polyclonal antiradixin antibody was purchased from Upstate technology(CST, U.S.A). Mouse monoclonal anti-E-Cadherin,anti-TSP-1 antibodies, and anti-MMP9 antibodies werepurchased from Cell Signaling Technology (Beverly,MA). The mouse monoclonal antibody to GAPDH waspurchased from Santa Cruz Biotechnology (Santa Cruz,CA). The secondary antibodies were purchased from SaierBiotechnology (Tianjin, China).RNA interferenceA radixin (GeneBank, No. L02320) shRNA sequencewas inserted into the pSIL-GFP lentivirus RNAiexpression system. The shRNA-containing vectors weretransfected together into 293T cells with pHelper1.0 andthe lentiviral helper plasmid pHelper 2.0 to generatethe respective lentiviruses. Viral stocks were collectedfrom the transduced 293T cells and were used to infectU251 cells. The radixin nonsense shRNA sequence wasACTACCGTTGTTATAGGTG, and the radixin shRNAsequence was GACGACAAGTTAACACCTAAA. ThemRNA and protein levels were measured 72 h after cellswere infected.Quantitative RT-PCRTotal cellular RNA was extracted with M-MLVRTase (Promega, Madison, WI) according to themanufacturer’s protocols. The resulting cDNA wasused for PCR using the SYBR-Green Master PCRMix (Applied Biosystem, Carlsbad, CA) in triplicates.Primers for qRT-PCR were as follows: radixin forwardprimer: CGAGGA AGAACGTGTAACCGAA; radixinreverse primer: TCTTGGTTTCATCTCTGGCTTG;E-Cadherin (GeneBank, No. Z13009) forward primer:C G G G A AT G C A G T T G A G G AT C ; E - C a d h e r i nreverse primer: AGGATGGTGTAAGCGATGGC;TSP-1 (GeneBank, No. X04665) forward primer:9806Asian Pacific Journal of Cancer Prevention, Vol 15, 2014TGGAACTATGGGCTTGAGAAAAC; TSP-1 reverseprimer: CACTGATGCAAGCACAGAAAAGA;MMP-9 (GeneBank, NM 004994.2) forward primer:TCAGGGAGACGCCCATTTC; and MMP-9 reverseprimer: TGGCAGGGTTTCCCATCAG. PCR and datacollection were performed on the PCR System (Takara,Shiga, Japan). All quantitation was normalized to anendogenous GAPDH control. GAPDH forward primer:AACGGATTTGGTCGTATTG; GAPDH reverse primer:GGAAGATGGTGATGGGATT. The relative quantitationvalue for each target gene compared to the calibrator forthat target is expressed as 2-ΔΔCt, ΔΔCt (Ct1-Ct2)(Ct3-Ct4) (Ct is the mean threshold cycle differences afternormalizing to GAPDH).Western blottingWestern blotting was performed as previouslydescribed (Guo et al., 2009). For blotting, cell lysates(30 μg protein) were separated by SDS-polyacrylamidegel electrophoresis (PAGE) using NuPAGE 4%-12% BisTris gels, SDS running buffer, and NuPAGE antioxidant(Invitrogen). Proteins were transferred to polyvinylidenefluoride membrane (Invitrogen) using NuPAGE transferbuffer supplemented with NuPAGE antioxidant in 30%methanol. Standard proteins used were Magic-Mark XP Western protein standard (Invitrogen) for molecularweight estimations. The membrane was blocked for 1h in room temperature in blocking solution (5% bovineserum albumin in TBS containing 0.05% Tween) andsubsequently incubated with primary antibodies, Rabbitpolyclonal anti-radixin (CST, U.S.A), and antibodiesagainst E-Cadherin, TSP-1, MMP9, and GAPDH inblocking solution overnight at 4 C. The membrane wasrinsed thoroughly in TBS containing 0.05% Tween andincubated for 1 h in room temperature with horseradishperoxidase conjugated secondary antibodies in blockingsolution. After subsequent rinsing, immunoreactive bandswere detected using Super Signal West PICO ChemilumiSubstrate (Thermo Fisher Scientific, Waltham, MA, USA)and an LAS3000 CCD camera (Fujifilm, Tokyo, Japan).Immunocytochemical analysisCell pieces were dyed according to the manufacturer’sprotocols. The pieces were fixed in 4% paraformaldehyde30 min in a 4 C refrigerator. Antibodies were thenadded, colored after being re-dyed by hematoxylin, anddehydrated by descending alcohol concentrations. Thepieces were observed and documented with an AxioImager A1 (Zeiss, Oberkochen, Germany).Immunohistochemical analysisImmunohistochemical assay was performed aspreviously described (Persson et al., 2010). The sectionswere de-waxed in xylene, dehydrated through gradedalcohol concentration, and washed in tris buffer (pH7.2). For antigen retrieval, the sections were immersedin citrate buffer (pH 6.2) at 90 C for 1h using a waterbath and then incubated with 3% hydrogen peroxide inmethanol for 15 min to block endogenous peroxidaseactivity. After washing twice in tris buffer, sections wereincubated 3-4 h at room temperature separately with
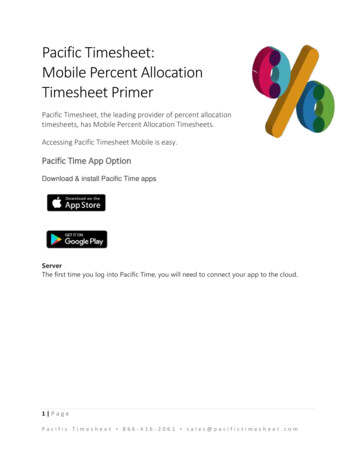
5Radixin Knockdown by RNA Interference Suppresses Human Glioblastoma Cell Growth in Vitro and in Vivopolyclonal antibody. Following washing in tris buffer,the sections were sequentially incubated with biotinylatedlink secondary antibody for 30 min in each. The sectionswere washed twice again in tris buffer and incubated withthe chromogen diaminobenzidine-tetrahydrochloridesubstrate, followed by counterstaining in hematoxylin.The sections were mounted in DPX mountant. Parallelcontrol slides were run with all batches, including positiveand negative controls.MTT assayMTT assay was performed as previously described(Chen et al., 2010). Viable cells (2 105 cells/ml) wereplated into 96-well plates (100 μl complete medium/well)and cultured at 37 C in 5% CO2. At different time points,MTT reagent (5 mg/ml) (DingGuo Biotech, Beijing,China) was added (20 μl per well) and incubated at 37 Cfor 4h. The reaction was stopped with 100 μl DMSO andthe optical density was determined at OD570 nm on amulti-well plate reader.Cell Cycle AssayCell cycle analysis was performed by flow cytometryas described previously (Meng et al., 2010). 5 105 cellswere plated in 60 mm dishes and cultured for 2 days. Cellswere collected by trypsinization, fixed with 95% ethanol,washed with PBS, resuspended in 1 mL of 0.01M PBSwith RNase and 50 μg/mL propidium iodide, incubated for20 min in the dark at room temperature, and analyzed byflow cytometry using a FACS Calibur (Becton Dickinson,Bedford, MA).Apoptotic cell detectionCells were stained with fluorescein isothiocyanate(FITC) labeled annexin-V and propidium iodide (PI) stainsimultaneously to discriminate intact cells (annexin-/PI-)from apoptotic cells (annexin /PI-) and necrotic cells(annexin /PI ). A total of 2.0 105 cells were washed twicewith ice-cold PBS and incubated for 30 min in a bindingbuffer (1 μg/ml PI and 1 μg/ml FITC labeled annexin-V),respectively. FACS analysis for annexin-V and PI stainingwas performed by flow cytometer.Adhesion assay96-well plates were coated 1 h at 37 C with 20 μg/mlof fibronectin (FN) and blocked with 0.5% BSA for 1 h atroom temperature. Then, 4 105 /ml cells were seeded ineach well and incubated at 37 C for 30 min in 5% CO2.After incubation, unbound cells were removed alongwith the culture medium. Remaining adherent cells werecolored for 15 min with 0.5% violet crystal and fixed with20% methanol. After washing with PBS, adherent cellswere lysed with 1% SDS. Adhesion was quantified withspectrophotometric optic density measurement at 550 nm.Invasion assayInvasion assay was performed as previously described(Albini et al., 1987). In short, cells were harvested andwashed with serum-free medium. 1 105 cell suspensionwas then added into the matrigel transwells, with 200μl of culture medium containing 10ng/ml ECM as thechemoattractant in the lower chamber. Cells that invadedthrough the matrigel membrane were stained and countedafter 24h incubation at 37 C.Tumor growth in nude miceFemale BALB/c nude mice (obtained from BeijingHuaFuKang Biotechnology Co., LTD, Beijing; bodyweight, 15 to 17g) were bred under specified pathogenfree conditions (26 C, 70% relative humidity, and a12h light/12h dark cycle) in a germ-free environmentwith free access to food and water. The logarithmicallygrowing cells were trypsinized and resuspended inD-Hanks solution. 2 106 cells in 0.1 ml were injectedsubcutaneously into the mice’s left flanks. Experimentaland control groups had at least 10 mice each. Tumorswere measured twice weekly with microcalipers, and thetumor volume was calculated according to the formula:V (volume) LW2 π/6, where “L” represents the greatestlength and “W” represents the perpendicular width (Butleret al., 1986). The animals were sacrificed by cervicaldislocation under light ether anesthesia after 4 weeks. Thetumors were excised and weighed. Part tumor specimenswere fixed in 4% formaldehyde, embedded in paraffin, andcut in 4 μm sections for immunohistochemical analysis(IHC). Parts were kept at -80 C refrigeration and usedfor Quantitative RT-PCR (qRT-PCR) and Western blotdetection (WB). All procedures met national guidelinesfor care and use of laboratory animals and were approvedby the Institutional Animal Care and Use Committee ofCapital Medical University, Beijing, China.Quantification of tumor microvessel density and in situTUNEL assay for apoptotic cellsTumor microvessel densities (MVD) were quantifiedby anti-CD31 immunohistochemistry as previouslydescribed (Singh et al., 2010). Apoptotic cells in tumorsamples were identified by terminal deoxyribonucleotidyltransferase dUTP nick end fluorescein labeling (TUNEL)assay according to the manufacturer’s instructions (In situCell Death Detection Kit, Roche, Indianapolis, IN). Thenumber of apoptotic cells was evaluated by counting thepositive (brown-stained) cells in 10 random fields ( 200).Statistical analysisEach experiment was performed three to four times. Alldata were expressed as mean SD. Statistical analysis wasperformed with SPSS 13.0 software (SPSS Inc., Chicago,IL). Comparisons between groups were conducted usingone-way analysis of variance (ANOVA) or Student’s t test.P values 0.05 were considered statistically significant.ResultsRadixin mRNA and protein expression in glioblastomaU251 cellsTo investigate radixin expression, we compared theglioblastoma U251 with normal glial cells by IHC, WB,and qRT-PCR, as shown in Figure 1. There are radixinexpressions in normal glial cells at both the mRNA andprotein levels and higher radixin expression levels inglioblastoma cells compared to normal glial cells.Asian Pacific Journal of Cancer Prevention, Vol 15, 20149807
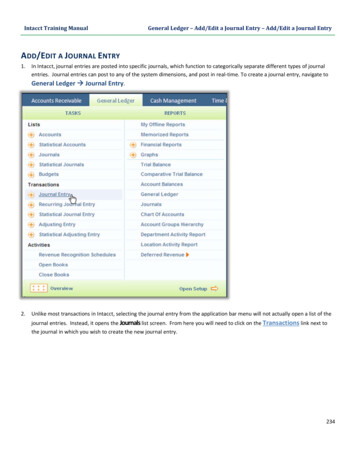
Jun-Jie Qin et alFigure 1. Radixin mRNA and Protein Expressions inGlioblastoma U251 Cells. Higher radixin expression inglioblastoma U251 cells compared with normal astrocyte glialcells. (A) The numbers of staining cells were increased markedlyon U251 cells compared with normal astrocyte glial cells; (B)WB shows radixin protein level in U251 cells is higher whencompared; (C) The radixin mRNA level is higher compared withnormal astrocyte glial cells. Results represent the mean SD ofthe experiments. *p 0.05Figure 2. Efficient shRNA Delivery into GlioblastomaU251 Cells and Stable Suppressing of Radixin in U251Cells. (A) High efficiency of transduction with fluorescentshRNA (green) in U251 cells was easily identified 48h posttransduction. Transduction comparison of the two groups. Mock:blank control; Lenti-radixin: radixin shRNA; (B) The radixinprotein level was down-regulated stably by radixin shRNA inU251 cells; (C) The radixin mRNA level was down-regulatedstably by radixin shRNA in U251 cells. Results represent themean SD of two independent experiments.*p 0.05Reduction of radixin mRNA and protein expression byshRNA in vitroTo investigate radixin’s biological role in U251 cells,we knocked down radixin transcript by employing shRNAtechnology. ShRNA to radixin was constructed into pSILGFP vector using a lentivirus transduction system, radixinmRNA and protein expression by shRNA in vitro alsoreduced, as shown in Figure 2.9808Asian Pacific Journal of Cancer Prevention, Vol 15, 2014Figure 3. Down-Regulation of Radixin InhibitsProliferation, Promotes Apoptosis, Inhibits theAdhesion and Invasion of U251 Cells. (A) U251 cellproliferation was inhibited when treated with radixin shRNAby MTT assay; (B) The proportion of U251 cells increased withG1 phase as a consequence of decreased radixin expression;(C) Down-regulation of radixin inhibits glioblastoma U251 celladhesion. The number of adherent living cells was significantlylower in the lenti-radixin group than in the mock group andcontrol group; (D) Down-regulation of radixin promotesglioblastoma U251 cell apoptosis; (E) Matrigel-coated transwellchambers were used to detect cell invasion and representativefields were photographed; (F) The decrease in the numbers ofinvasive cells in the Lenti-radixin group compared to those ofthe Mock or Control group was statistically significant. Resultsrepresent the mean SD of three independent experiments. *p 0.05Radixin down-regulation inhibited glioblastoma U251cell proliferationUsing radixin cell down-regulation, we first testedU251 cell growth changes using the MTT assay. Resultsshowed a significant cell growth decrease and indicatedthat cell proliferation was inhibited after transduction withradixin shRNA (Figure 3A). The flow cytometry assayshowed that the proportion of cells in the G1 phase wassignificantly increased and those in the G2 and S phaseswere decreased after transduction with radixin shRNA(Figure 3B). These results indicated that radixin couldenhance the U251 cell growth ability in vitro.Radixin down-regulation promoted glioblastoma U251cell apoptosisTo determine the effects of radixin’s down-regulationon the radixin-induced apoptosis in U251 cells, theapoptosis rate was evaluated by flow cytometry analysis.As shown in Figure 3D, the radixin-induced apoptosiseffects were investigated in cells treated with radixinshRNA as well as cells in blank controls and cells treatedwith nonsense shRNA. We found a significantly elevatedapoptosis percentage in cells transfected with radixinshRNA compared to blank control cells (p 0.045), whilethere was no significant difference between blank controlsand cells transfected with nonsense shRNA (p 0.05).
5Radixin Knockdown by RNA Interference Suppresses Human Glioblastoma Cell Growth in Vitro and in VivoFigure 4. Down-Regulation of Radixin Reduces TumorGrowth, Increases Apoptosis and Decreases MicrovesselDensity of Glioblastoma in Vivo. Glioblastoma cells(Control, Mock, or Lenti-radixin) were subcutaneously injectedinto the left flank regions of nude mice. (A) Tumor volumes weremeasured every three days with a caliper and were calculated;using the formula π/6 (smaller diameter) 2 (larger diameter).(B) Tumor volumes from day 0 to day 28 (n 10). Lenti-radixingroup growth was significantly reduced (p 0.05) comparedwith Control or Mock group; (C) Representative photographsof decreases in microvessel density from the tumor sectionsexamined by TUNEL assay; (D) The data present the averagenumber of apoptotic cells SD; (E) Immunohistochemicalstaining for microvessels with anti-CD31. The representativepictures are shown at 200 magnification; (F) Microvesseldensity was quantitated microscopically with a 5 5 reticle gridat 400 magnification. The values are mean SD.*p 0.05Radixin down-regulation inhibited the adhesion andinvasion of glioblastoma U251 cellsIn the cell adhesion in vitro experiment, the number ofadherent living cells was significantly lower in the radixinshRNA group (Lenti-radixin) than in the nonsense shRNAgroup (Control) and blank control group (Mock) (Figure3C). These results suggest that radixin shRNA induces theloss of cell adhesion to the matrix. Since two critical stepsare involved in metastasis, adhesion and invasion, we nextexamined whether radixin can affect U251 cell invasionactivity by the matrigel invasion assay. Cell invasiveactivity was also dramatically decreased in the Lentiradixin cells compared with the Mock or Control cells.The average cell number invading to the lower chamberfor 24h was 54.33 7.51/HPF in the radixin shRNA cells,compared to 126.33 8.62/HPF in the Mock cells or111.33 10.69/HPF in the Control cells. The quantitativeanalysis showed that the number of radixin shRNA cellsinvading to the lower chamber was decreased by 57.01%compared to the Mock cells (p 0.05), while there was nosignificant difference between Mock and Control cells(p 0.05) (Figure 3E, 3F).Radixin down-regulation resulted in reduced tumor growthand angiogenesisTo specifically determine radixin’s role in tumorFigure 5. Suppressed Radixin mRNA and Proteinin Lenti-radixin Group in Vivo. (A) Representativephotographs of the tumor sections examined by microscopy.Immunohistochemical staining for radixin. The representativepictures are shown at 400 magnification; (B) The radixin proteinlevel was down-regulated by radixin shRNA in vivo; (C) Theradixin mRNA level was down-regulated by radixin shRNAin vivo. Results represent the mean SD of three independentexperiments. *p 0.05development, Lenti-Radixin, Control, or Mock cells wereinjected into the left flank regions of nude mice (n 10/group). All of the mice developed tumors. Four weeksafter tumor injection, tumor volumes were measured.Subcutaneous tumors formed from Lenti-Radixin cellswere significantly smaller compared with the Mockor Control cells (Control, 155.85 50.25mm3; Mock,116.36 19.25 mm3; Lenti-Radixin, 56.18 6.40 mm3)(Figure 4A, 4B). Additionally, Lenti-Radixin tumors hadsignificantly increased numbers of TUNEL-positive cells(p 0.05) (Figure 4C, 4D).One possible mechanism for decreased tumor growthis attenuated neovascularization. To determine whetherradixin down-regulation could potentially disrupt theneovascularization, we examined vascularity in tumors.Staining on Lenti-Radixin tumor sections showed a 4.7fold decrease in the number of blood vessels comparedwith Mock or Control tumors (Figure 4E, 4F).Reduction of radixin mRNA and protein expression byshRNA in vivoLenti-Radixin tumors had significantly decreasedRadixin expression compared to Mock or Control tumors(p 0.05) (Figure 5). Similar results were found in vitro.Thrombospondin-1 (TSP-1), MMP9, and E-Cadherin wereregulated in Lenti-radixin U251 cellsTo determine the mechanism(s) by which radixinregulates tumor growth and progression, we examinedAsian Pacific Journal of Cancer Prevention, Vol 15, 20149809
Jun-Jie Qin et al9810Asian Pacific Journal of Cancer Prevention, Vol 15, 20146.312.856.351.133.131.3Newly diagnosed without treatmentChemotherapyNoneRemissionPersistence or recurrenceNewly diagnosed with treatmentNewly diagnosed without treatment2002; McClatchey, 2003; Chen et al., 2012). Mice lackingradixin are characterized by hepatocyte apical microvillibreakdown, which ultimately results in mild liver injurysimilar to human conjugated hyperbilirubinemia in DubinJohnson syndrome (Kikuchi et al., 2002). High ERMexpression levels were observed in many tumor cell lines,such as the breast carcinoma and osteosarcoma cell line(Khanna et al., 2004; Revillion et al., 2008). However, itsrole and mechanisms remain elusive.Our results showed that radixin was expressed in100.0astrocytes in the central nervous system (CNS), radixin100.0expressionin glioblastoma U251 cells compared6.3is higher10.120.3with normal astrocytes cells, and knocking down radixin25.0 and survival.75.030.075.0resulted in an inhibition of cell proliferationAdditionally, we observed decreased Lenti-radixin U251cell adhesion and invasion.We established experimental46.856.3mice models and showed that radixin knock-down54.250.0regulated primary tumor growth,angiogenesis,and50.031.3Figure 6. Thrombospondin-1 (TSP-1), E-Cadherin,30.0progression. Hence, our study’s results suggest anand MMP9 Were Regulated in Lenti-radixin U251important correlation between radixin expression andCells. (AB) The TSP-1 and E-Cadherin mRNA levels were upregulated by radixin shRNA in U251 cells; (C) MMP9 mRNA25.0glioblastoma cancer growth and invasion. So, increased25.0radixin expression 38.0is associated with enhanced cell growthlevels were down-regulated by radixin shRNA in U251 cells; (D)31.331.330.0and poor malignant GBM prognosis.23.7The TSP-1 and E-Cadherin protein levels were up-regulated byIncreased cell proliferation and decreased cell deathradixin shRNA in U251 cells. MMP9 protein levels were downregulated by radixin shRNA in U251 cells. Results represent the 0play a pivotal role in tumor progression. Any decreased 0mean SD of two independent experiments. *p 0.05tumor growth of radixin-suppressed cells may be due totheir decreased response to cell proliferation, adhesion,potential radixin-regulated molecules. As we know,survival, or invasion. The present in vitro data revealsE-cadherin plays a central role in heterotypic epithelialthat suppressing radixin results in a significant decreasecell-cell adhesion and maintaining epithelial cell colonyin proliferation. Another important finding of this studyintegrity (Takeichi, 1993). TSP-1 is a potent inhibitor ofwas the enhanced apoptosis of radixin-suppressed cells.neovascularization that limits tumor growth (Castle etAn apoptotic property of these cells was observed both inal., 1997). MMP9 is an important protease of the matrixvitro and in tumor tissues. Our results are consistent withmetalloproteinase (MMPs) family in glioblastoma biology,previous finding in hippocampal pyramidal cells (Pagliniwhich can degrade the extracellular matrix (ECM). MMP9et al., 1998). The observed effects of radixin silencing onis up-regulated in glioma cell lines and human specimensthe GBM cell transformation indicate a role for radixin in(Uhm et al., 1997; Forsyth et al., 1998; Forsyth et al.,regulating tumor metastasis and progression. Our results1999). MMPs are overexpressed not only by gliomaare consistent with previous finding in Ezrin (Curto andcell lines but also by the tumor vasculature, suggestingMcClatchey, 2004).a relevant role in angiogenesis induction and regulationCell invasion into the surrounding tissue is a multi(Liotta et al., 1991; Hanahan and Folkman, 1996). In thisstep action that requires cell-cell contact, cell motility,study, Western blot analysis and quantitative RT-PCRand extracellular matrix (ECM) degradation by matrixrevealed that TSP-1 and E-Cadherin were dramaticallymetalloproteinases (MMPs). Malignant tumor cellsup-regulated but MMP9 was dramatically down-regulateddisplay varying degrees of resistance to detachment,in cells transfected with radixin shRNA (Figure 6). Thosegenerating cell-extracellular matrix interactions relatedresults suggest that TSP-1 and E-Cadherin expressionto the adhesion complex. This property contributes todown-regulation and MMP9 expression up-regulationtumorigenesis and metastasis (Frisch and Francis, 1994;might participate in radixin-mediated tumor growth andChen et al., 2012). Our in vitro study showed significantprogression.differences in adhesion and invasion between radixinshRNA and control cells. Radixin is a cytoskeletal proteinthat might affect the cytoskeletal element assembly atDiscussionthe cytoplasmic face of the membrane and the nuclearRadixin is a member of the ERM (ezrin-radixinskeleton, which would facilitate cell invasion. Thesemoesin) family and shares the common membraneresults agree with another report where ERM stablebinding N-terminal FERM domain with band-4.1knockdown by shRNA reduced in vitro human SGCfamily members (Turunen et al., 1994). By organizing7901 cell migration and invasion (Ou-Yang et al., 2011).membrane-cytoskeleton-associated complexes andAngiogenesis is another essential step for tumor growthcreating specialized membrane domains, the ERMand metastasis. Our immunohistochemistry results supportproteins regulate cellular activities like survival, adhesion,that primary tumor vasculature contributed to in vivoand migration/invasion, all of which are important duringdifferences between Lenti-radixin and nonsense shRNAtumor development and progression (Bretscher et al.,control tumors.
5Radixin Knockdown by RNA Interference Suppresses Human Glioblastoma Cell Growth in Vitro and in VivoE-cadherin was first discovered in 1995 by Berx (Berxet al., 1995). It is a calcium-dependent cell-cell adhesionmolecule with pivotal roles in epithelial cell behavior,tissue development, and cancer growth suppression(Stemmler, 2008; van Roy and Berx, 2008; Sun et al.,2013). TSP-1 is a potent neovascu
gel electrophoresis (PAGE) using NuPAGE 4%-12% Bis-Tris gels, SDS running buffer, and NuPAGE antioxidant (Invitrogen). Proteins were transferred to polyvinylidene fluoride membrane (Invitrogen) using NuPAGE transfer buffer supplemented with NuPAGE antioxidant in 30% methanol. Standard proteins used were Magic-Mark