Transcription
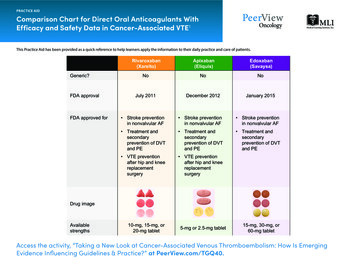
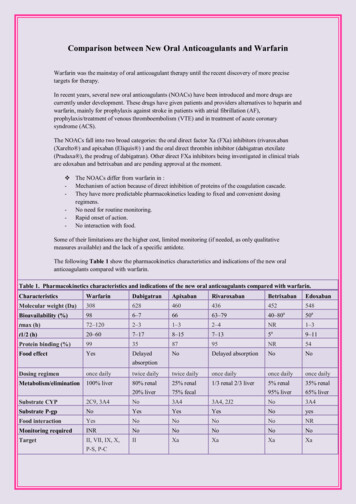
Comparison between New Oral Anticoagulants and WarfarinWarfarin was the mainstay of oral anticoagulant therapy until the recent discovery of more precisetargets for therapy.In recent years, several new oral anticoagulants (NOACs) have been introduced and more drugs arecurrently under development. These drugs have given patients and providers alternatives to heparin andwarfarin, mainly for prophylaxis against stroke in patients with atrial fibrillation (AF),prophylaxis/treatment of venous thromboembolism (VTE) and in treatment of acute coronarysyndrome (ACS).The NOACs fall into two broad categories: the oral direct factor Xa (FXa) inhibitors (rivaroxaban(Xarelto ) and apixaban (Eliquis ) ) and the oral direct thrombin inhibitor (dabigatran etexilate(Pradaxa ), the prodrug of dabigatran). Other direct FXa inhibitors being investigated in clinical trialsare edoxaban and betrixaban and are pending approval at the moment. The NOACs differ from warfarin in :- Mechanism of action because of direct inhibition of proteins of the coagulation cascade.- They have more predictable pharmacokinetics leading to fixed and convenient dosingregimens.- No need for routine monitoring.- Rapid onset of action.- No interaction with food.Some of their limitations are the higher cost, limited monitoring (if needed, as only qualitativemeasures available) and the lack of a specific antidote.The following Table 1 show the pharmacokinetics characteristics and indications of the new oralanticoagulants compared with warfarin.Table 1. Pharmacokinetics characteristics and indications of the new oral anticoagulants compared with RivaroxabanBetrixabanEdoxabanMolecular weight (Da)308628460436452548aBioavailability (%)986–76663–7940–8050atmax (h)72–1202–31–32–4NR1–3at1/2 (h)20–607–178–157–1359–11Protein binding (%)99358795NR54Food effectYesDelayedabsorptionNoDelayed absorptionNoNoDosing regimenonce dailytwice dailytwice dailyonce dailyonce dailyonce dailyMetabolism/elimination100% liver80% renal20% liver25% renal75% fecal1/3 renal 2/3 liver5% renal95% liver35% renal65% liverSubstrate CYP2C9, 3A4No3A43A4, 2J2No3A4Substrate P-gpNoYesYesYesNoyesFood interactionYesNoNoNoNoNRMonitoring requiredINRNoNoNoNoNoTargetII, VII, IX, X,P-S, P-CIIXaXaXaXa
AntidoteYesNoNoNoNoNoTypical effective doseINR guided150 mg or220 mg once2.5 bid (VTEprophylaxis)*10 mg once daily(VTE prophylaxis)*Indevelopment30 mg(VTEdaily (VTEprophylaxis)*15 mg twice daily(1–21) followed by75 mg or 150mg twice20 mg once daily(DVTdaily (AF) ‡treatment/preventionof recurrentprophylaxis)VTE) 20 mg oncedaily (AF)‡Approved indicationsApproved forVTE preventionand treatment ofthethromboemboliccomplicationsassociated withAF and cardiacvalvereplacement,and secondaryprevention afterMIApproved forVTEpreventionafter electivehip or kneereplacementin adults andforprevention ofstroke andsystemicembolism inpatients withnonvalvularApproved forVTEpreventionafter electivehip or kneereplacementin adults.Strokepreventionand systemicemolizationinnonvalvularAFApproved for VTEprevention afterelective hip or kneereplacement inadults, forprevention of strokeand systemicembolism in patientswith nonvalvularAF, and fortreatment of acuteDVT and preventionof VTE recurrenceHas notbeenapprovedyetOnlyapproved inJapan forVTEprophylaxisjointreplacementAFAdapted from Poulsen et al. [2012], Lip et al. [2012] and Perez et al. [2013].ACS, acute coronary syndrome; AF, atrial fibrillation; CYP, cytochrome P450; DVT, deep vein thrombosis; INR, internationalnormalized ratio; MI, myocardial infarction; NR, not reported; P-C, protein C; P-gp, P glycoprotein; P-S, protein S; t½, terminalelimination half-life; tmax, time to reach maximal plasma concentration; VTE, venous thromboembolism.*Approved dose for VTE prevention in adult patients undergoing elective hip or knee replacement surgery. Approved dose for treatment of acute DVT and prevention of recurrent DVT and PE.‡Approved dose for prevention of stroke and systemic embolism in adult patients with nonvalvular AF.Renal Clearance of warfarin and NOACsNo dosage adjustment for warfarin is necessary in renal impairment. However, patients with renal failurehave an increased risk of bleeding complications; so monitor closely.Given that all the NOACs are partially excreted by the kidneys, careful watch over the creatinine clearance(CrCl) will avoid excessive drug accumulation in the body.Dabigatran serves as a substrate for P-gp and although CYP3A4 has no role in its metabolism, changes in itsbioavailability can be expected if other drugs that are strong P-gp inhibitors, such as amiodarone, verapamil,ketoconazole, quinidine or clarithromycin, or inducers such as rifampicin and St John's wort are used.
However, since a third of rivaroxaban is cleared by the kidneys, it has been approved for use in patients withsevere renal insufficiency (CrCl level between 15 and 29 ml/min). On the other hand, CYP3A4 plays a role inthe metabolism of rivaroxaban and drug–drug interactions are quite likely if inhibitors and inducers ofCYP3A4 are concomitantly administered.The following Table 2 shows the approved dosing for NOACs according to indication and renal function.Table 2. Approved dosing according to indication and renal function.Drug and indicationRenal function classificationNormalCLcr 90ml/minMildCLcr60–89ml/minModerateCLcr 30–59 ml/minSevere CLcr15–29 ml/minEnd-stage renaldisease CLcr 15ml/minAtrialfibrillation/EMA150 mgtwicedaily150 mgtwicedaily110 mgtwice dailyContraindicatedContraindicatedAtrial fibrillation/FDA150 mgtwice150 mgtwice150 mgtwice daily75 mg twicedaily ContraindicateddailydailyVTE prophylaxis jointreplacement220 mgoncedaily220 mgoncedaily150 mgonce dailyContraindicatedContraindicatedVTE treatment (not150 mg150 mg150 mgContraindicatedContraindicatedyet approved)twicedailytwicedailytwice dailyAtrial fibrillation20 mgoncedaily20 mgoncedaily15 mg oncedaily15 mg oncedailyContraindicatedVTE prophylaxis joint10 mgoncedaily10 mgoncedaily10 mg oncedailyUse with cautionContraindicated15 mgtwice15 mgtwice15 mgtwice15 mg twicedaily/15 mgContraindicateddaily/20mg oncedaily/20mg oncedaily/15mg onceonce dailydailydailydaily5 mgtwicedaily5 mgtwicedaily5 mg twicedailyDabigatranRivaroxabanreplacementVTE treatmentApixabanAtrialfibrillation/EMA/FDA2.5 mg twicedailyContraindicated
VTE prophylaxis jointreplacement2.5 mgtwicedaily2.5 mgtwicedaily2.5 mgtwice dailyUse with cautionContraindicated30 mg30 mg30 mg lydailyEdoxaban*VTE prophylaxis jointreplacementAdapted from Poulsen et al. [2012] and Turpie et al. [2012].*Only approved in Japan. Dose based on pharmacokinetic modeling, not on randomized control trials.CLcr, creatinine clearance; EMA, European Medicines Agency; FDA, US Food and Drug Administration; VTE,venous thromboembolism.Emergent Reversal of Warfarin and NOACsUrgent reversal of the old as well as new oral anticoagulants is of paramount importance and is often required inemergency surgery, or bleeding associated with intracranial hemorrhage.While NOACs have several advantages over warfarin, the major disadvantage, rather, a challenge is the lack ofspecific antagonists in perioperative care and bleeding management. Emergent reversal of NOACs is mandatoryin cases of life-threatening bleeding or when emergency surgical intervention warrants correction of coagulationprocesses.Figure1 illustrates the management of an elevated INR in patients taking warfarin and Figure 2 illustrates themanagement guideline for bleeding while taking dabigatran, rivaroxaban or apixaban.Figure 1: Management of an elevated INR in patients taking warfarin
Figure 2: Management guideline for bleeding while taking dabigatran, rivaroxaban or apixabanAdapted from Baumann Kreuziger et al. [2012].CLcr, creatinine clearance; ECT, ecarin clotting time; NOAC, new oral anticoagulant; PCC,prothrombin complex concentrate; rFVIIa, recombinant activated factor VII; TT, thrombin time.For life-threatening bleeding complications with dabigatran treatment, hemodialysis and hemofiltrationcould be options as dabigatran is dialyzable due to its relatively low (35%) plasma proteinbinding. However, dialysis is not suitable for rivaroxaban because of its high (95%) proteinbinding. Currently, there is no specific antidote for any of these agents.There is no place for the use of vitamin K or fresh frozen plasma (FFP) in the reversal of these agents.Prothrombin complex concentrate (PCC) contains factors II, VII, IX and X or factor viii inhibitorbypass activity (FEIBA) containing activated clotting factors II, VII, IX and X may be partiallyeffective in overcoming the effect of dabigatran and also rivaroxaban.The following Table 3 shows the actions to evaluate and nonspecific reversal agents to use with thenew oral anticoagulants.
Table 3. Actions to evaluate and nonspecific reversal agents to use with the new oral anticoagulants.DrugTherapeutic optionsDabigatranStop treatment with OACsHaemodialysisPCC (25 U/kg, repeat if needed)rFVIIa (90 μg/kg)RivaroxabanStop treatment with OACsPCC (25 U/kg, repeat if needed)FEIBA (50 IE/kg, max 200 IE/day)rFVIIa (90 μg/kg)ApixabanStop treatment with OACsPCC (25 U/kg, repeat if needed)FEIBA (50 IE/kg, max 200 IE/day)rFVIIa (90 μg/kg)EdoxabanStop treatment with OACsPCC (25 U/kg, repeat if needed)FEIBA (50 IE/kg, max 200 IE/day)rFVIIa (90 μg/kg)Adapted from Miesbach and Seifried [2012].The therapeutic options are not validated by large-scale trials.OAC, oral anticoagulant; PCC, prothrombin complex concentrate; rFVIIa, recombinant activated factor VII.Periprocedural Management of Patients on Warfarin and Chronic NOACsTherapyThe challenge in periprocedural management of patients receiving anticoagulant therapy focuses on the needto balance the risk of thromboembolism (in case of anticoagulation interruption) against the risk of bleedingduring the procedure (in case of anticoagulation continuation).Traditionally, the use of periprocedural bridging therapy with heparin, either unfractionated heparin or lowmolecular-weight heparin (LMWH), reduce the risk of periprocedural thromboembolism by allowing forcontinued anticoagulation during temporary discontinuation of warfarin for an elective procedure or surgery.Figure 3 below shows an overview of perioperative management of warfarin therapy and heparin bridgingbefore and after surgery/procedure.
Figure 3: overview of perioperative management of warfarin therapy and heparin bridgingbefore and after surgery/procedureThis research was originally published in Blood. Douketis JD. Perioperativemanagement of patients who are receiving warfarin therapy: an evidence -basedand practical approach. Blood 2011; 117:5044. Copyright 2011 AmericanSociety of Hematology.Thus, for the time being, the usual, although empirical, approach includes elective interruption of NOACstherapy before surgery, with timing of anticoagulant discontinuation depending on the half-life of theanticoagulant, the patient's renal function and the surgical risk of bleeding .
Table 4 summarizes recommendations regarding timing of discontinuation of the main NOACs beforestandard and high-risk bleeding procedures.High-bleeding risk procedures including cardiac surgery, neurosurgery, abdominal surgery or proceduresrequiring spinal anesthesia.Table 4. Discontinuation guide for the main new oral anticoagulants, before standard and highrisk bleeding procedures.Renal functionDabigatranRivaroxabanApixaban(CLcr ml/min )StandardHigh riskStandardHigh riskStandardHigh risk ofrisk ofofrisk ofofrisk ofbleedingbleedingbleedingbleedingbleedingbleeding 5024 h2–4 days24 h3 days24–36 h3 days30–5048 h4 days48 h3 days48 h4 days 302–5 days 5 days3 days4 daysAdapted from van Ryn et al. [2010], Spyropoulos and Douketis [2012] and Baumann Kreuziger et al. [2012].CLcr, creatinine clearance.Timing of resumption of NOACs after surgery depends on bleeding risks and the dose used, as summarizedin Table 5.Table 5. Postoperative resumption of new oral anticoagulants: a suggested managementapproach.DrugLow bleeding risk surgeryHigh bleeding risk surgeryDabigatranResume on day after surgery (24 hResume 2–3 days after surgery (48–72 hpostoperative), 150 mg twice dailypostoperative), 150 mg twice daily*Resume on day after surgery (24 hResume 2–3 days after surgery (48–72 hpostoperative), 20 mg once dailypostoperative), 20 mg once daily Resume on day after surgery (24 hResume 2–3 days after surgery (48–72 hpostoperative), 5 mg twice dailypostoperative), 5 mg twice daily RivaroxabanApixabanAdapted from Spyropoulos and Douketis [2012].Standard dosages shown, consider dose reduction in cases of renal function impairment (creatinine clearance 50ml/min).*For patients at high risk for thromboembolism, consider administering a reduced dose of dabigatran (e.g. 110–150mg once daily) on the evening after surgery and on the following day (first postoperative day) after surgery.Efficacy and risk of bleeding for warfarin versus NOACsA large meta-analysis assess the safety and efficacy of NOACs versus warfarin for the prevention of stroke inpatients with atrial fibrillation indicated NOACs protect against stroke or systemic embolism betterthan warfarin and compare favorably on safety in patients with atrial fibrillation (AF). In contrast, the risk ofgastrointestinal bleeding was greater on the NOACs.NOACs are effective for the long term treatment for VTE with reduced risk with VTE related deathscompared with placebo but similar efficacy to warfarin.
The cost of warfarin versus NOACs in JordanDrugDosage formWarfarin( Orfarin )Dabigatran (Pradaxa )3 mg, 5 mg tablet75,110,150 mgcapsuleRivaroxaban(Xarelto )15, 20 mg tabletNumber oftablet,capsule100 tablets60 capsulesCost(JD)3.71 , 5.2101.3928 tablets92.66References :1- Douketis.J. Perioperative management of patients who are receiving warfarin therapy: anevidence-based and practical approach. Blood 2011; 117:50442- Vílchez.J.et al. Safety of New Oral Anticoagulant Drugs. Ther Adv in Drug Safe. 2014;5(1):820.3 - Woodhouse. C.et al. Systematic Review: The New Oral Anticoagulants. FrontlineGastroenterol. 2013;4(3):213-218.4- New Oral Anticoagulant Drugs. Personalized Medicine. 2013;10(5):419-422.5- Vallakati.A. COMPARATIVE EFFICACY AND SAFETY OF NEW ORALANTICOAGULANTS VERSUS WARFARIN FOR LONG-TERM TREATMENT OF VENOUSTHROMBOEMBOLISM: A META-ANALYSIS. J Am Coll Cardiol.2014;63(12 S):. doi:10.1016/S0735-1097(14)62097-06- 'New' Oral Anticoagulant Stroke-Protection Benefits in AF Cut Across Subgroups in MetaAnalysis. December 05, pared by: Pharm.D : Eshraq Al-abweenyThe Supervisor OF Drug Information Office / Jordan University of Science andTechnologyExt:23233E.mail : dio@just.edu.jo7/4/2014
Comparison between New Oral Anticoagulants and Warfarin Warfarin was the mainstay of oral anticoagulant therapy until the recent discovery of more precise targets for therapy. In recent years, several new oral anticoagulants (NOACs) have been introduced and more drugs are currently under development.